What Is Dalton S Law Of Partial Pressures Dalton s law also called Dalton s law of partial pressures states that in a mixture of non reacting gases the total pressure exerted is equal to the sum of the partial pressures of the individual
Jan 30 2023 nbsp 0183 32 Dalton s Law or the Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture What is Dalton s Law Dalton s law of partial pressures is a gas law which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures exerted by
What Is Dalton S Law Of Partial Pressures

What Is Dalton S Law Of Partial Pressures
https://cdn1.byjus.com/wp-content/uploads/2018/11/chemistry/2017/08/06095757/Partial-Pressure-1.jpg
/Dalton-s_law_of_partial_pressures-56a1338a3df78cf7726858f7.png)
What Is Dalton s Law Of Partial Pressures
https://fthmb.tqn.com/XPa0_hZfU0IgNvhXYrEKIlZAwEI=/3076x1172/filters:fill(auto,1)/Dalton-s_law_of_partial_pressures-56a1338a3df78cf7726858f7.png

Dalton s Law Of Partial Pressure Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/12/Daltons-Law-of-Partial-Pressure.png
Jun 25 2023 nbsp 0183 32 This law states that in a mixture of two or more gases the total pressure is the sum of the partial pressures of all the components The partial pressure of a gas is the pressure that Dalton s law of partial pressure states that in a mixture of two or more non reacting gases the sum of the partial pressures of each gas is equal to the overall pressure of the gas mixture A
Apr 10 2025 nbsp 0183 32 Subtract water vapor pressure from total pressure to get partial pressure of gas A P A 1 03 atm 1 atm 0 03 atm 2 The law of partial pressures also applies to the total number Jan 9 2019 nbsp 0183 32 Dalton s law of partial pressures is used to determine the individual pressures of each gas in a mixture of gases The total pressure of a mixture of gases is equal to the sum of
More picture related to What Is Dalton S Law Of Partial Pressures
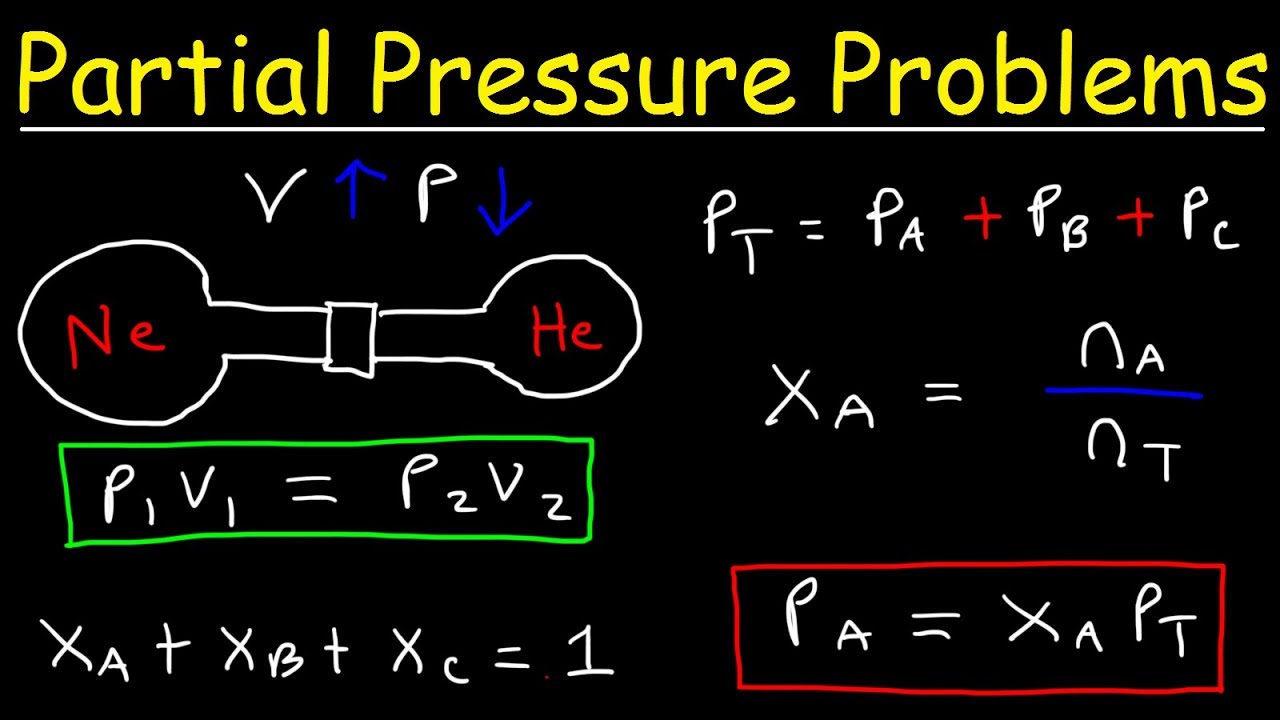
Dalton s Law Of Partial Pressure Problems Mole Fraction Chemistry Gas
https://i.ytimg.com/vi/J7YRwU7IV8Q/maxresdefault.jpg

Dalton s Law Of Partial Pressure YouTube
https://i.ytimg.com/vi/Lc0Gbuvu3Nc/maxresdefault.jpg

Dalton s Law Of Partial Pressures YouTube
https://i.ytimg.com/vi/y5-SbspyvBA/maxresdefault.jpg
Dalton s Law says that summing the individual pressures of each gas gives the total pressure of the mixture In effect a gas mixture acts as if there is no mixture there s just gas particles And Dalton s law the statement that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual component gases The partial pressure is the pressure that
May 6 2019 nbsp 0183 32 In 1801 Dalton formulated a law now known as Dalton s law of partial pressures which states that The total pressure of a mixture of gases is just the sum of the pressures that May 27 2024 nbsp 0183 32 Dalton s Law states that in a mixture of non reacting gases the total pressure exerted is equal to the sum of the partial pressures of individual gases The partial pressure of

Dalton s Law Of Partial Pressure States Of Matter Physical
https://www.logiota.com/upload/img/898878718-10055452020.jpg
What Is Dalton s Law Of Partial Pressure
https://search-static.byjusweb.com/question-images/aakash_pdf/99996899492-0-0
What Is Dalton S Law Of Partial Pressures - The law of partial pressures states that at a given temperature the total pressure of a gaseous mixture is equal to the sum of the partial pressures exerted by each of the gases making up the