Dalton S Law Of Partial Pressure Formula Dalton s Law Formula Dalton s law of partial pressures can be mathematically expressed as follows begin array l P total sum i 1 n p i end array or P total P 1 P 2 P 3 P n Where P total is the total pressure exerted by the mixture of gases
Jun 25 2023 nbsp 0183 32 The partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself Partial pressure is represented by a lowercase letter p Dalton s law of partial pressures is most commonly encountered when a gas is collected by displacement of water as shown in Figure 2 Dec 7 2021 nbsp 0183 32 Dalton s Law Formula The formula for Dalton s law states that the pressure of a gas mixture is the sum of the partial pressures of its component gases PT P1 P2 P3 Here P T is the total pressure of mixture and P 1 P 2 etc
Dalton S Law Of Partial Pressure Formula
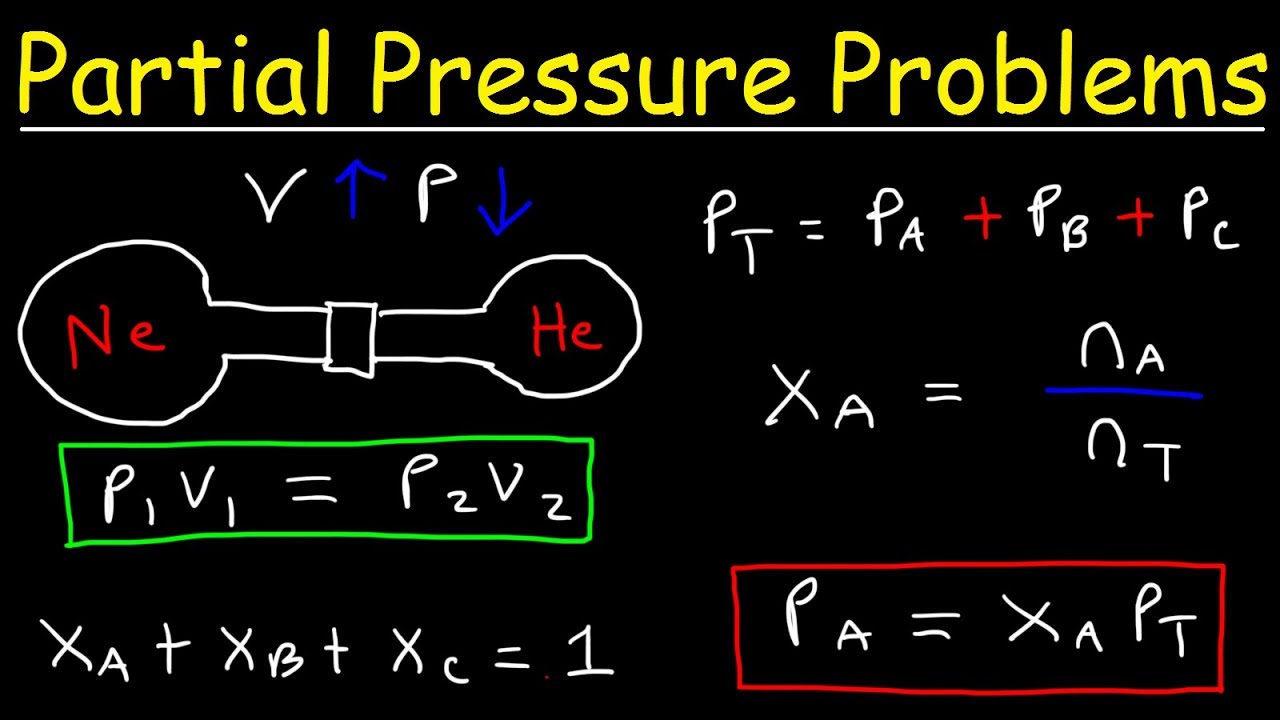
Dalton S Law Of Partial Pressure Formula
https://i.ytimg.com/vi/J7YRwU7IV8Q/maxresdefault.jpg

Dalton s Law Of Partial Pressure Formula Examples Video Lesson
https://study.com/cimages/videopreview/5.52_101795.jpg
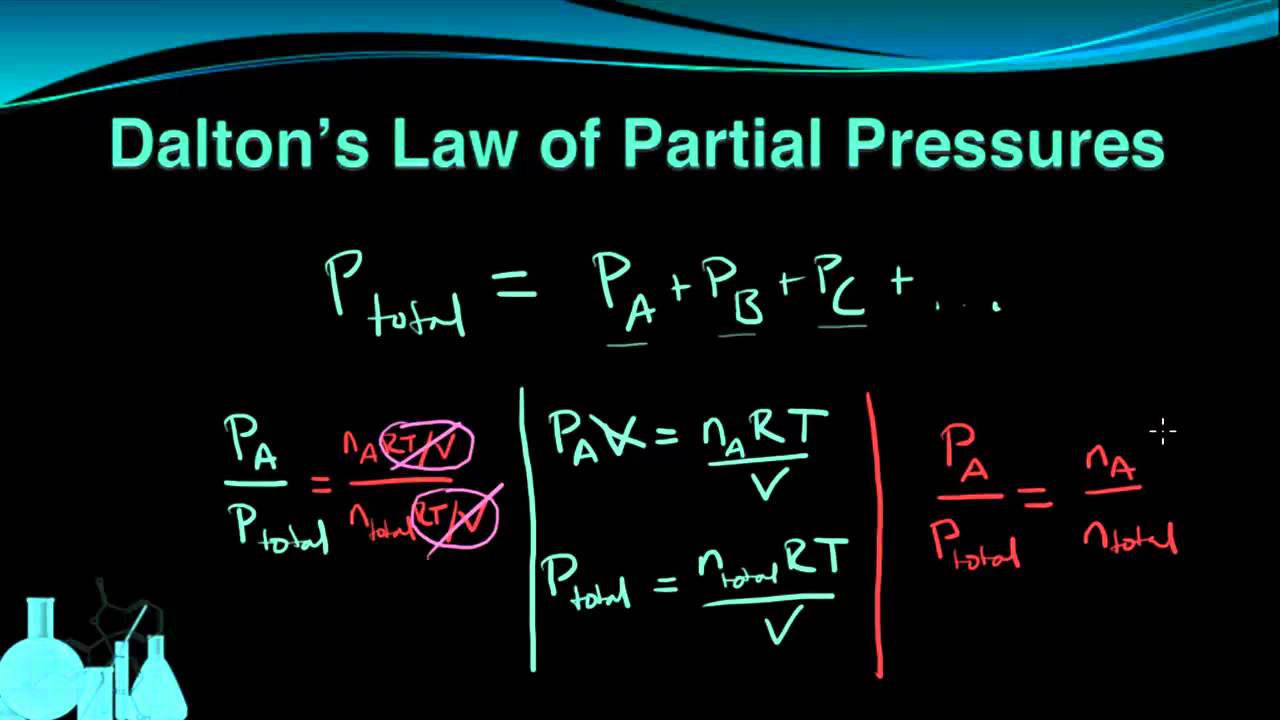
Chemistry 7 6 Dalton s Law Of Partial Pressures YouTube
http://i.ytimg.com/vi/gvXMg3J5j_Y/maxresdefault.jpg
Dalton s law also called Dalton s law of partial pressures states that in a mixture of non reacting gases the total pressure exerted is equal to the sum of the partial pressures of the individual gases 1 Dalton s law of partial pressure states that in a mixture of two or more non reacting gases the sum of the partial pressures of each gas is equal to the overall pressure of the gas mixture A gas s partial pressure is the pressure it would exert on the walls if it were the only gas in a container 1 4
May 27 2024 nbsp 0183 32 The formula for calculating partial pressure is Pi Xi 215 Ptotal where Pi is the partial pressure of the gas i Xi is the mole fraction of gas i and Ptotal is the total pressure of the mixture Dec 13 2023 nbsp 0183 32 The partial pressure of each gas in a mixture is proportional to its mole fraction The pressure exerted by each gas in a gas mixture its partial pressure is independent of the pressure exerted by all other gases present
More picture related to Dalton S Law Of Partial Pressure Formula

Dalton s Law Of Partial Pressures
https://s3.studylib.net/store/data/008945788_1-4373a625d0b30da8b7e4a825f1d285b5-768x994.png
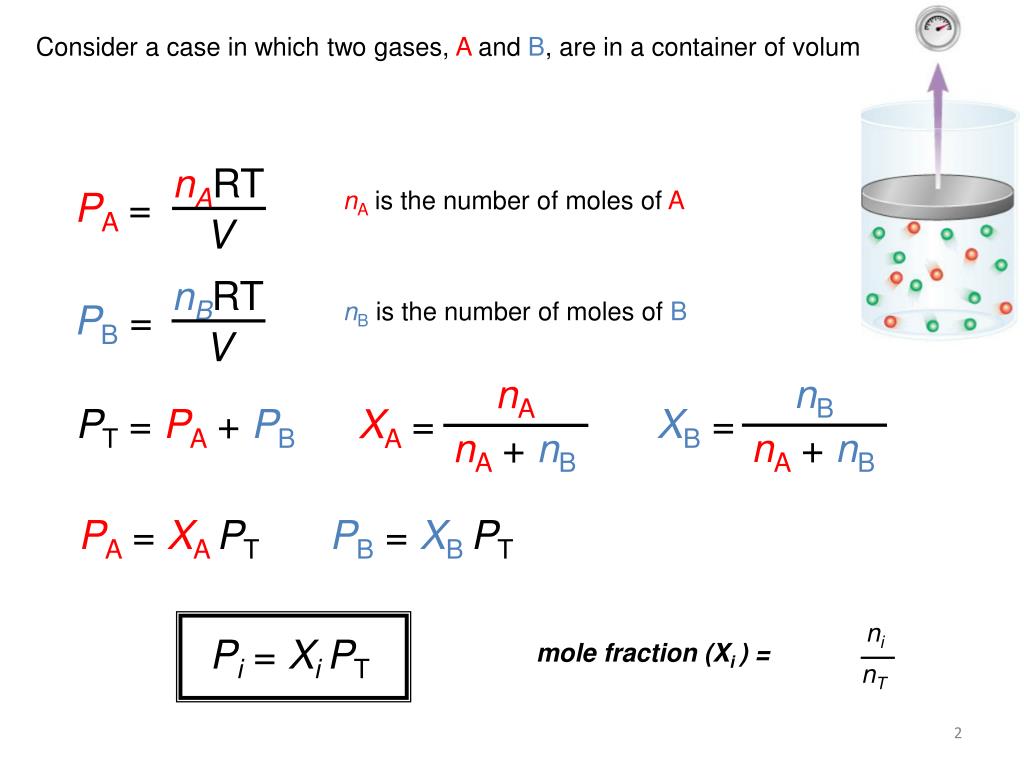
PPT Dalton s Law Of Partial Pressures PowerPoint Presentation Free
https://image3.slideserve.com/6592770/slide2-l.jpg

Dalton s Law Of Partial Pressure Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/12/Daltons-Law-of-Partial-Pressure.png
Learn how to use Dalton s Law of partial pressure equation the partial pressure formula to determine the pressures of gases and how it relates to mole fraction Dalton s law states that the total pressure of a gas mixture is the sum of the partial pressures of each individual gas
Dalton s law of partial pressures Pt P1 P2 says that the total pressure of a gas mixture is the sum of the partial pressures of constituent gases Jul 14 2024 nbsp 0183 32 Dalton s law states that The total pressure exerted on a container s walls by a gas mixture is equal to the sum of the partial pressures of each separate gas It can also be illustrated with an equation total pressure p1 p2 pn where p1 p2 and so on up to pn represent the partial pressure of each gaseous component

PPT Dalton s Law Of Partial Pressures PowerPoint Presentation Free
https://image.slideserve.com/283092/dalton-s-law-of-partial-pressures-n.jpg
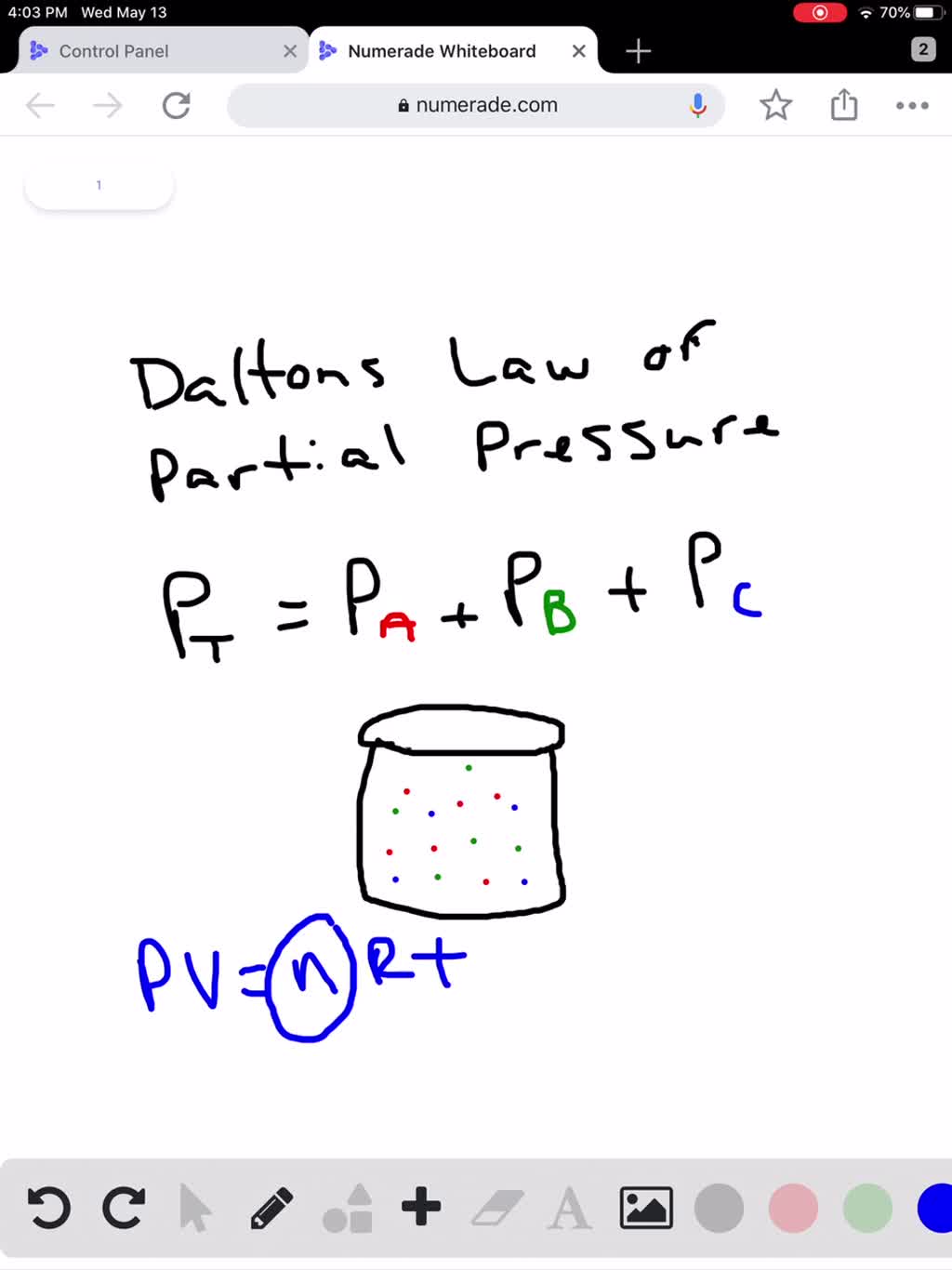
SOLVED How Does Dalton s Law Of Partial Pressures Help Us With Our
https://cdn.numerade.com/previews/59759064-52a3-429a-a18d-e94b649d49be_large.jpg
Dalton S Law Of Partial Pressure Formula - Dalton s law also called Dalton s law of partial pressures states that in a mixture of non reacting gases the total pressure exerted is equal to the sum of the partial pressures of the individual gases 1