Dalton S Law Of Partial Pressures Dec 7 2021 nbsp 0183 32 Dalton s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas English scientist John Dalton observed the behavior of gases in 1801 and published the gas law in 1802
Jan 30 2023 nbsp 0183 32 Dalton s Law or the Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture Dalton s law also called Dalton s law of partial pressures states that in a mixture of non reacting gases the total pressure exerted is equal to the sum of the partial pressures of the individual gases 1
Dalton S Law Of Partial Pressures

Dalton S Law Of Partial Pressures
https://www.logiota.com/upload/img/898878718-10055452020.jpg

Dalton s Law Statement Formula And Example Problems
https://www.chemistrylearner.com/wp-content/uploads/2022/12/Daltons-Law.jpg

Dalton s Law Of Partial Pressure Ideal Gas Equation Gases And
https://i.pinimg.com/originals/1c/fe/fe/1cfefec6c29a474e1db6e88b185264a3.jpg
Jun 25 2023 nbsp 0183 32 This law states that in a mixture of two or more gases the total pressure is the sum of the partial pressures of all the components The partial pressure of a gas is the pressure that gas would exert if it occupied the container by itself The pressure of a gas in a gas mixture is termed the partial pressure Dalton s law of partial pressure says that the total pressure in a gas mixture is the sum of the individual partial pressures
Jan 9 2019 nbsp 0183 32 Dalton s law of partial pressures is used to determine the individual pressures of each gas in a mixture of gases What is Dalton s law of partial pressure How does it apply to gases Learn its equation along with a few solved problems
More picture related to Dalton S Law Of Partial Pressures
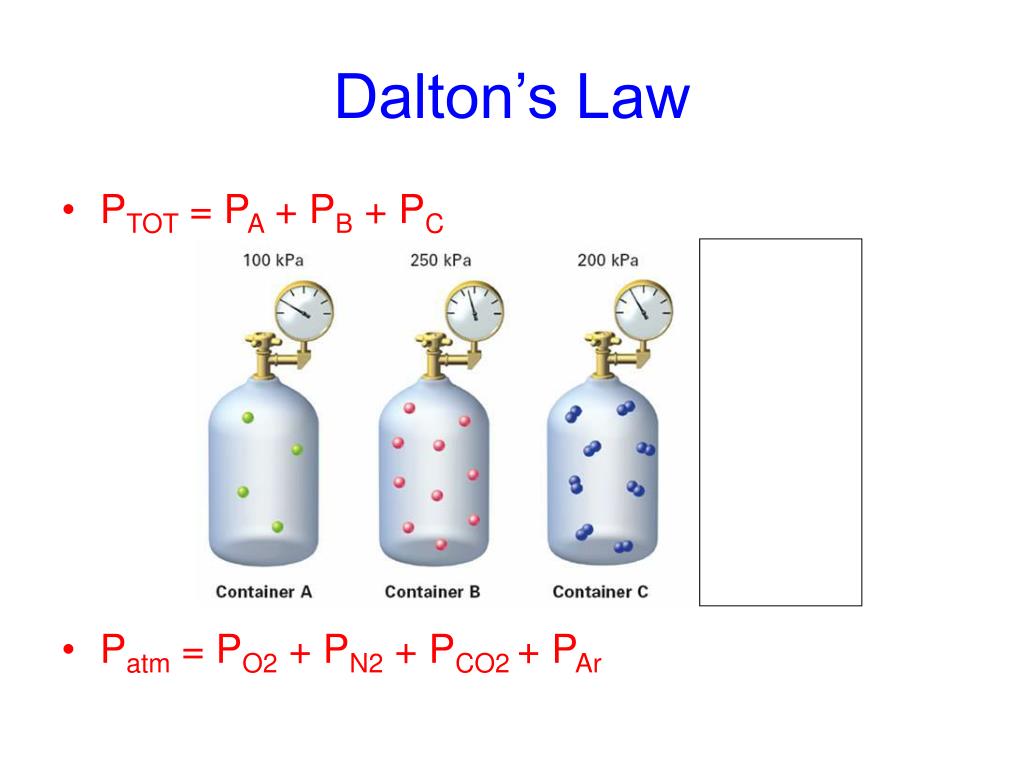
Dalton s Law Worksheets
https://image3.slideserve.com/6599100/dalton-s-law1-l.jpg
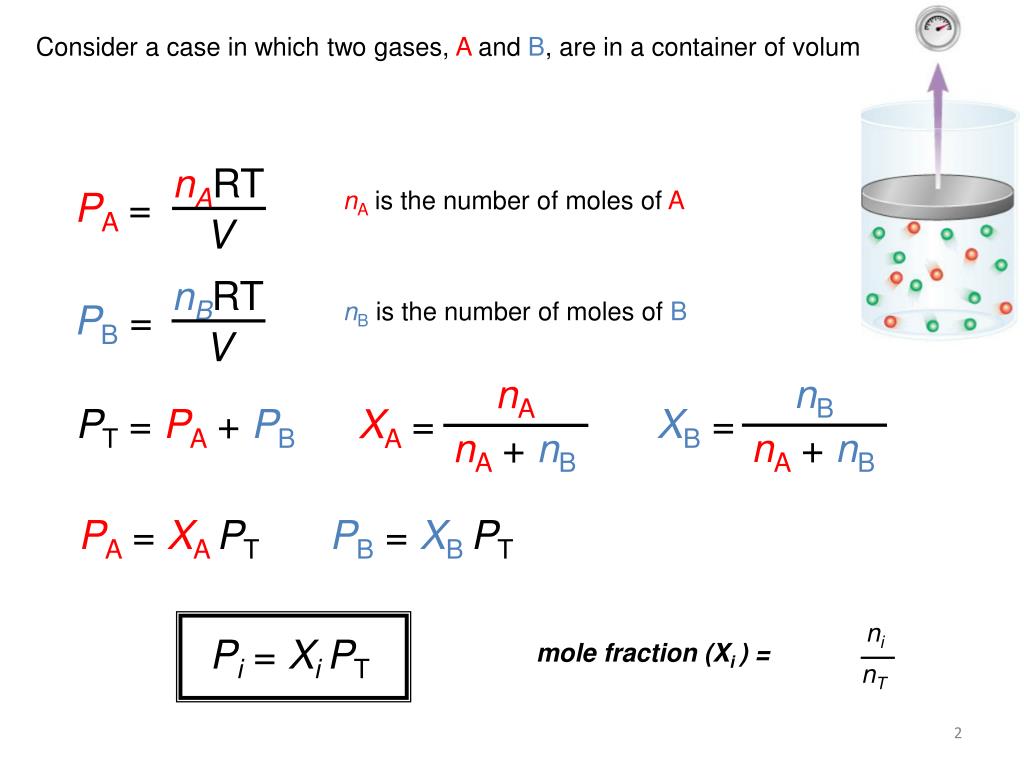
Dalton s Law Of Partial Pressure Worksheet With Answers
https://image3.slideserve.com/6592770/slide2-l.jpg

DALTON S LAW OF PARTIAL PRESSURES STATES OF MATTER 17 YouTube
https://i.ytimg.com/vi/rNxDFS_-wYU/maxresdefault.jpg
Dalton s law states that the total pressure exerted by the mixture of inert non reactive gases is equal to the sum of the partial pressures of individual gases in a volume of air This empirical law was observed by John Dalton in 1801 and is related to the ideal gas laws May 27 2024 nbsp 0183 32 Explore Dalton s Law of Partial Pressures its calculations applications and significance in chemistry and physics with practical examples
[desc-10] [desc-11]

Dalton s Law Of Partial Pressures
https://s3.studylib.net/store/data/008945788_1-4373a625d0b30da8b7e4a825f1d285b5-768x994.png

Dalton s Law Of Partial Pressures Explained YouTube
http://i.ytimg.com/vi/RqffPYOoxd8/maxresdefault.jpg
Dalton S Law Of Partial Pressures - [desc-14]