What Is Dalton S Law Of Partial Pressure Apex Dalton s Law or the Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture
What is Dalton s Law Dalton s law of partial pressures is a gas law which states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures exerted by each individual gas in the mixture Jun 5 2024 nbsp 0183 32 Dalton s Law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas in
What Is Dalton S Law Of Partial Pressure Apex

What Is Dalton S Law Of Partial Pressure Apex
https://sciencenotes.org/wp-content/uploads/2021/12/Daltons-Law-of-Partial-Pressure.png
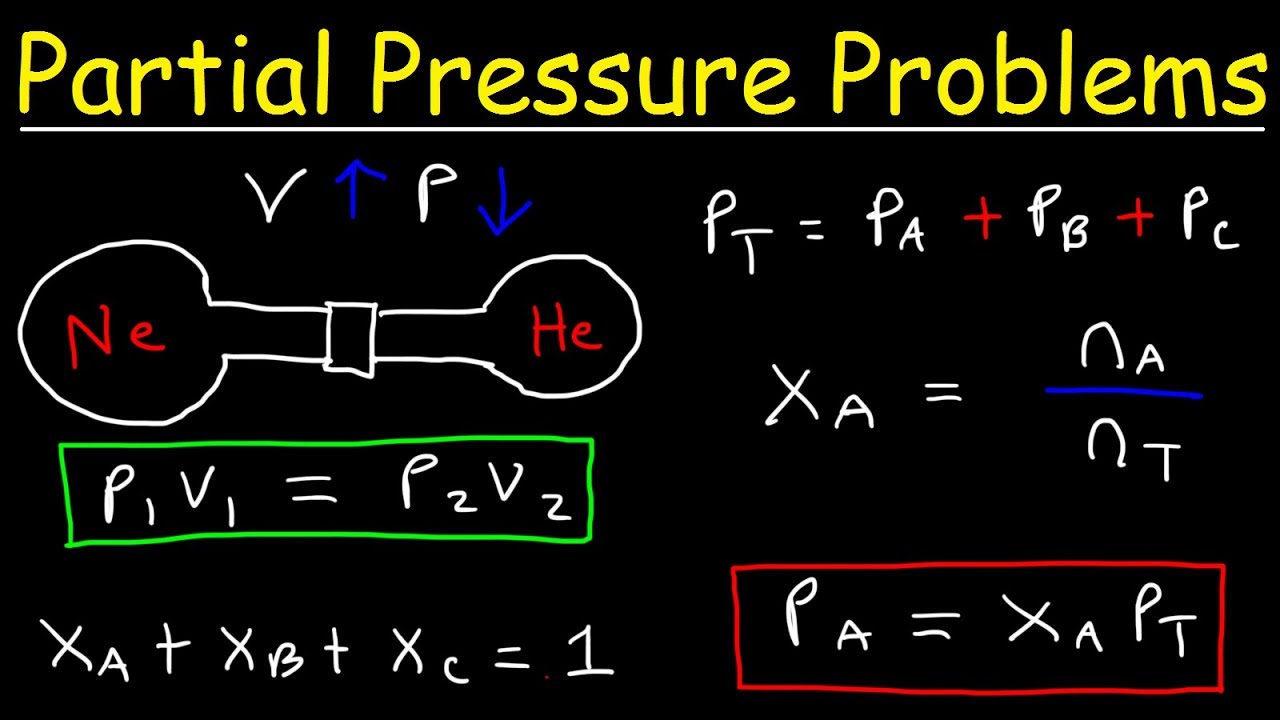
Dalton s Law Of Partial Pressure Problems Mole Fraction Chemistry Gas
https://i.ytimg.com/vi/J7YRwU7IV8Q/maxresdefault.jpg
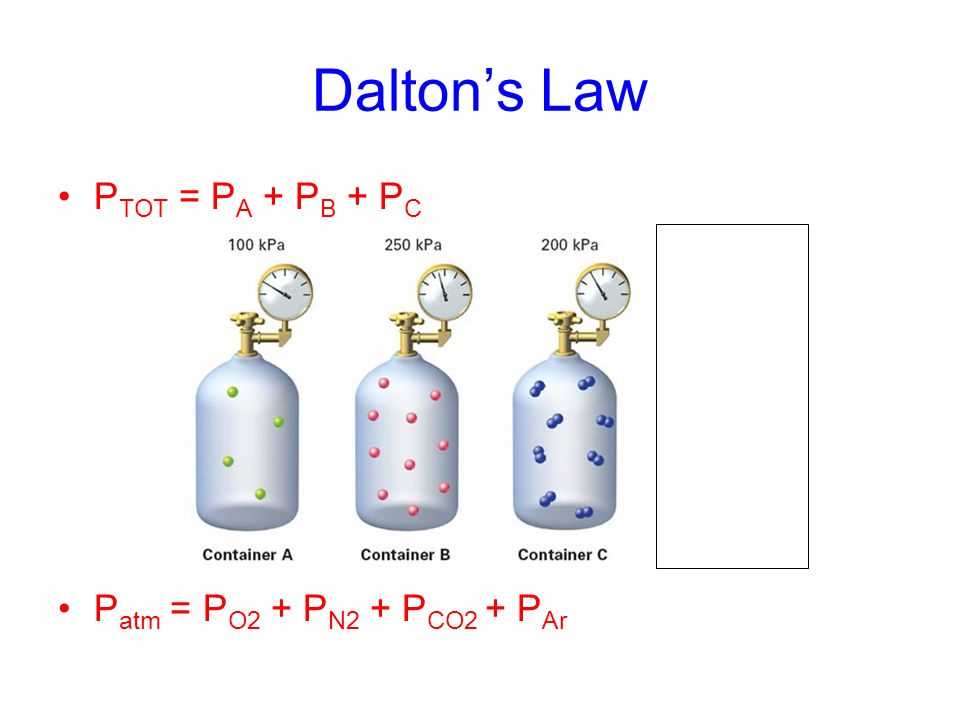
Master Dalton s Law Of Partial Pressures With This Answer Key
https://onelearningblog.com/wp-content/images/free-byffe-dalton's-law-of-partial-pressures-answer-key.jpg
Dalton s law of partial pressure first published by John Dalton in the year 1802 states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures exerted by each individual gas present in the mixture In the year 1801 John Dalton related the partial pressure of each gas with the total pressure of the gas at a constant temperature and volume using an ideal gas equation and formulated a law known as Dalton s law of partial pressure
May 6 2019 nbsp 0183 32 In 1801 Dalton formulated a law now known as Dalton s law of partial pressures which states that The total pressure of a mixture of gases is just the sum of the pressures that each gas would exert if it were present alone The following figure illustrates Dalton s law Apr 1 2025 nbsp 0183 32 Dalton s law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of all of the partial pressures of the component gases Dalton s law can be expressed with the following equation Ptotal P1 P2 P3
More picture related to What Is Dalton S Law Of Partial Pressure Apex
What Is Dalton s Law Of Partial Pressure
https://search-static.byjusweb.com/question-images/aakash_pdf/99996899492-0-0

State And Explain Daltons Law Of Partial Pressures How Is This Law
https://d1hj4to4g9ba46.cloudfront.net/questions/1928910_1865910_ans_bad6423f66d640ecb0d6db55e3eb3e18.jpg

Dalton s Law Of Partial Pressure YouTube
https://i.ytimg.com/vi/Lc0Gbuvu3Nc/maxresdefault.jpg
Dalton s law of partial pressure states that in a mixture of two or more non reacting gases the sum of the partial pressures of each gas is equal to the overall pressure of the gas mixture Dalton s Law or the Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture
The pressure exerted by each gas in a gas mixture its partial pressure is independent of the pressure exerted by all other gases present Consequently the total pressure exerted by a mixture of gases is the sum of the partial pressures of In this tutorial you will learn what partial pressure is how to find the partial pressure of a gas using the partial pressure formula and how Dalton s Law relates it to mole fraction

Daltons Law Of Partial Pressures Dalton s Law Science Flashcards
https://i.pinimg.com/736x/9e/93/8b/9e938ba615d7bf16d5cf5aad70f6a155.jpg

Dalton s Law Of Partial Pressure States Of Matter Physical
https://www.logiota.com/upload/img/898878718-10055452020.jpg
What Is Dalton S Law Of Partial Pressure Apex - In the year 1801 John Dalton related the partial pressure of each gas with the total pressure of the gas at a constant temperature and volume using an ideal gas equation and formulated a law known as Dalton s law of partial pressure