State Gay Lussac Law Of Gaseous Volume Gay Lussac s Law is a Gas Law which States that the Pressure of a Gas of a Given mass kept at a constant Volume Varies Directly with its Absolute Temperature
Jan 30 2023 nbsp 0183 32 This law formulated by Gay Lussac states that quot the ratio between the volumes of gaseous reactants and products can be expressed in simple whole numbers quot For example in the following reaction t he ratio of volumes of hydrogen chlorine and Gay Lussac s law usually refers to Joseph Louis Gay Lussac s law of combining volumes of gases discovered in 1808 and published in 1809 1 However it sometimes refers to the proportionality of the volume of a gas to its absolute temperature at constant pressure
State Gay Lussac Law Of Gaseous Volume

State Gay Lussac Law Of Gaseous Volume
https://hi-static.z-dn.net/files/d75/49478c8ad14655975fd0f5069ae25da6.jpg

Gay Lussac S Law Of Gaseous Volumes Class Chapter My XXX Hot Girl
https://static-images.findfilo.com/classroom/1675062133233_dmdphydx_331045.jpg
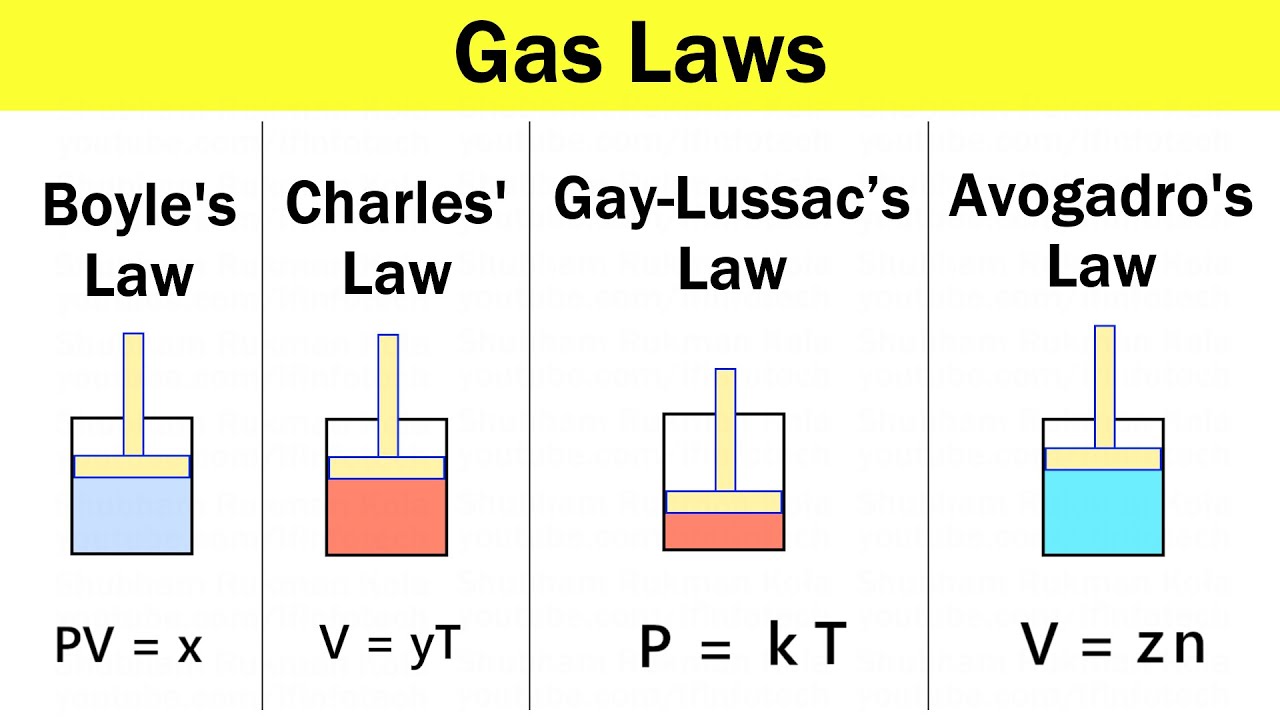
Gas Laws Boyle s Law Charles s Law Gay Lussac s Law And Avogadro s
https://i.ytimg.com/vi/YcldP6HPuF4/maxresdefault.jpg
Apr 15 2024 nbsp 0183 32 Gay Lussac s Law of gaseous volume states that when gases react to form a new gaseous product the ratio of the volume of gaseous reactant to the volume of gaseous product is a simple whole number ratio provided the reaction takes place at May 6 2024 nbsp 0183 32 Gay Lussac s law states that the pressure exerted by a gas varies directly with the absolute temperature of the gas if the mass of the gas is fixed and the volume is constant i e The pressure exerted by a gas is proportional to the temperature of the gas at constant volume
The Gay Lussac s law of gaseous volumes relates the pressures and volumes of gases to their temperatures and physical states The formula which is named after French physicist and chemist Joseph Gay Lussac is often used to determine the stoichiometric proportions of gases in chemical reactions Apr 1 2021 nbsp 0183 32 Gay Lussac s law or Amonton s law states that the absolute temperature and pressure of an ideal gas are directly proportional under conditions of constant mass and volume In other words heating a gas in a sealed container causes its pressure to increase while cooling a gas lowers its pressure
More picture related to State Gay Lussac Law Of Gaseous Volume

Define Gay Lussac s Law Of Gaseous Volumes Explain With One Suitable
https://haygot.s3.amazonaws.com/questions/1930618_1865983_ans_16c4206844014e35ac4d169ff60a92c6.jpg

Law Combining Volumes Gay Lussac Stock Vector Royalty Free 1711030981
https://www.shutterstock.com/shutterstock/photos/1711030981/display_1500/stock-vector-law-of-combining-volumes-gay-lussac-1711030981.jpg
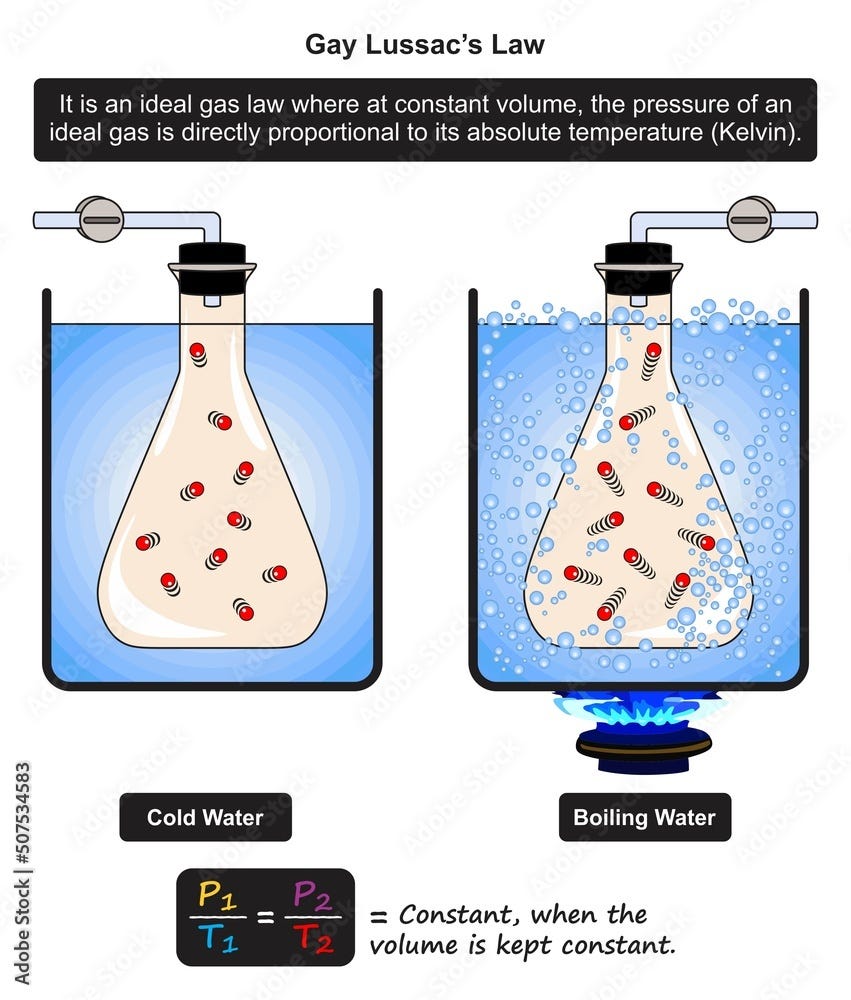
Gay Lussac s Law Teach Chemistry
https://miro.medium.com/v2/resize:fit:851/1*4kz9ayitzGA1w8CxG4vdzQ.jpeg
Aug 17 2023 nbsp 0183 32 Gay Lussac s Law sometimes known as the law of combining volumes is a fundamental principle in the field of chemistry According to Gay Lussac s Law a gas s pressure and temperature are both inversely correlated when kept Jun 25 2023 nbsp 0183 32 According to Gay Lussac s law for a given amount of gas held at constant volume the pressure is proportional to the absolute temperature Mathematically P T or P kGT or P T kG P T or P k G T or P T k G where kG is the appropriate proportionality constant
3 days ago nbsp 0183 32 The French chemist Joseph Gay Lussac 1778 1850 discovered the relationship between the pressure of a gas and its absolute temperature Gay Lussac s Law states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas when the volume is kept constant Gay Lussac s Law elucidates a critical relationship between pressure and temperature in a confined gas system The law asserts that at constant volume and mass the pressure of a gas is directly proportional to its absolute temperature

Statement l Gay Lussac s Law Of Gaseous Volumes States That When Gases C
https://static-images.findfilo.com/classroom/1665892732672_fufbxfea_1234642.jpg

1 12 Basic Concepts Of Chemistry Gay Lussac s Law Of Gaseous
https://i.ytimg.com/vi/UqgcFSxvoIE/maxresdefault.jpg
State Gay Lussac Law Of Gaseous Volume - The Gay Lussac s law of gaseous volumes relates the pressures and volumes of gases to their temperatures and physical states The formula which is named after French physicist and chemist Joseph Gay Lussac is often used to determine the stoichiometric proportions of gases in chemical reactions