Oxidation Number Worksheet And Answers One way of reflecting this is through changes in assigned oxidation numbers Oxidation numbers are real or hypothetical charges on atoms assigned by the following rules Atoms in elements are assigned 0 All simple monatomic ions have oxidation numbers equal to their charges e g all Group 1 ions are 1 all group 2 ions are 2 all the
Rule 1 The oxidation number of an element in its free uncombined state is zero for example Al s or Zn s This is also true for elements found in nature as diatomic two atom elements H2 O2 S8 Rule 2 The oxidation number of a monatomic one atom ion is the same as the charge on the ion for example Na S2 A monoatomic ion has an oxidation number equal to its charge For example the oxidation number of the oxygen in the oxide ion O 2 is 2 The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion Let s examine the oxidation numbers of some common elements Notice the periodic trend among the main group
Oxidation Number Worksheet And Answers
Oxidation Number Worksheet And Answers
http://www.unmisravle.com/wp-content/uploads/2018/03/pictures_oxidation_number_practice_worksheet_1.jpg
16 Best Images Of Organic Oxidation Reactions Worksheet Balancing
http://www.worksheeto.com/postpic/2012/11/oxidation-numbers-worksheet_202222.jpg

The 8 Rules Of Oxidation Numbers Wovo
https://picswovo.wovo.org/what_is_oxidation_number_in_chemistry.png
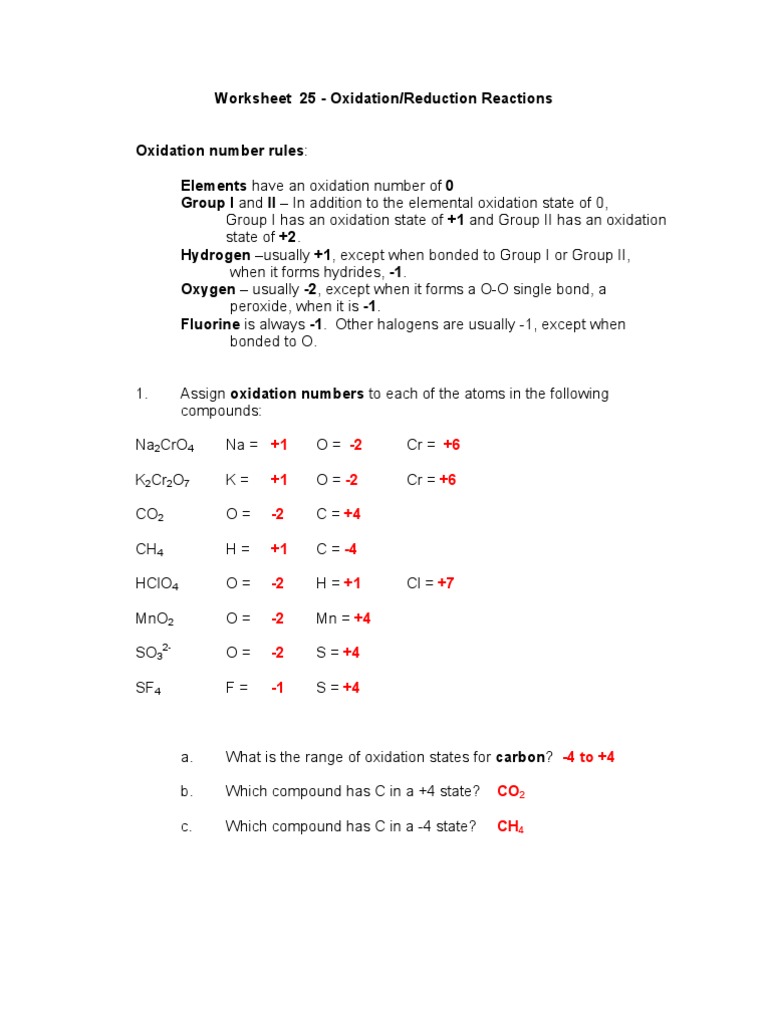
Oxidation Reduction Reactions Worksheet You should try to answer the questions without referring to your textbook If you get stuck try asking another group for help Describe the trend in oxidation number for main group elements as you move left to right across a row in the periodic table 11 3 The oxidation number of combined hydrogen is usually 1 4 The oxidation number of combined oxygen is usually 2 5 The sum of all oxidation numbers of atoms in a compound is zero 6 The sum of all oxidation numbers of atoms in an ion is equal to the charge on that ion No Question Answer 1 What is the oxidation number of the atom in bold
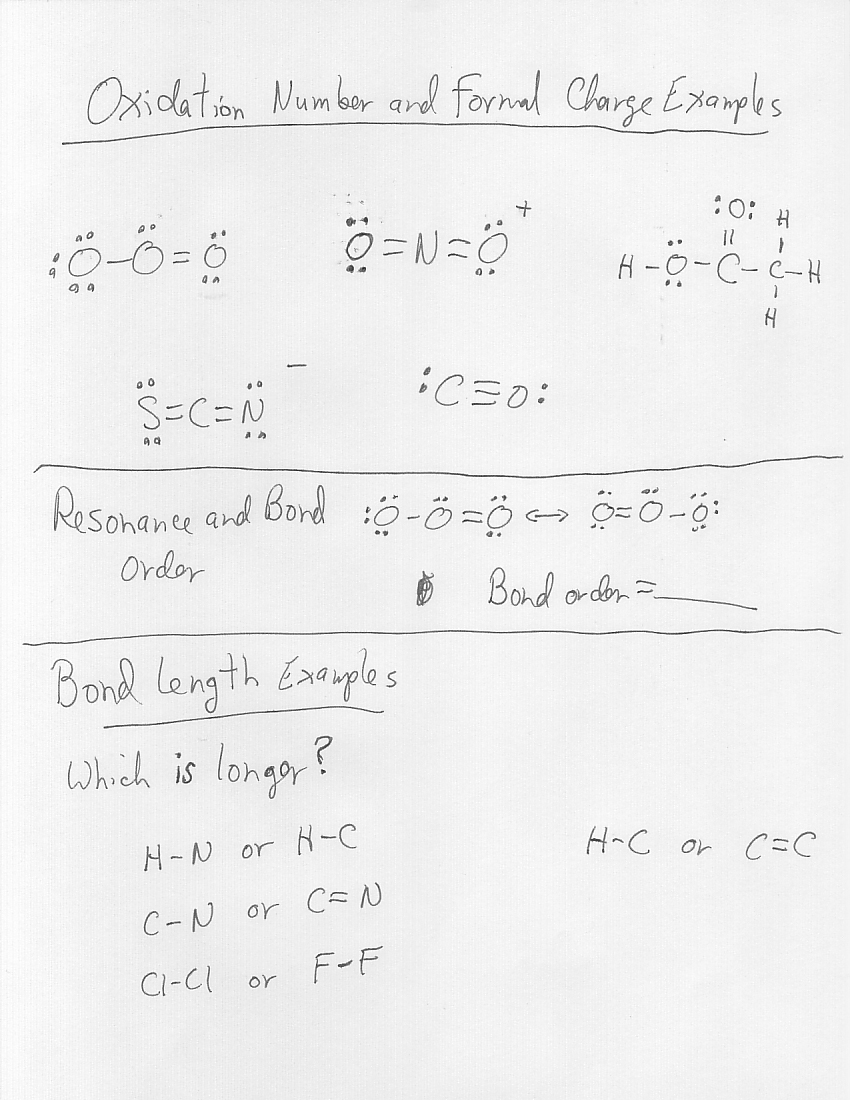
Worksheet 1 Rules for Assigning Oxidation Numbers 1 The oxidation number of any uncombined element is 0 2 The oxidation number of a monatomic ion equals the charge on the ion 3 The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion Chemistry Chapter 7 Worksheets Chemical Formulas and Nomenclature page 1 WORKSHEET 1 Determination of oxidation number or valence number Rules to apply 1 a The net charges on all molecules is zero therefore the sum of the positive charges equals the sum of the negative charges 2 a
More picture related to Oxidation Number Worksheet And Answers
Oxidation Reduction Worksheet doc
https://s2.studylib.net/store/data/015301580_1-e092699c030435ee2c166eccfa76c1db.png

Oxidation WS Chapter 1 Redox Reactions Practice Problems
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/d4b765cd5e13afcf4f91a2b8a172b16e/thumb_1200_1553.png
Solved What Is The Oxidation State Of The Oxygen Atoms In CO2 O2 And
https://www.coursehero.com/qa/attachment/13691469/
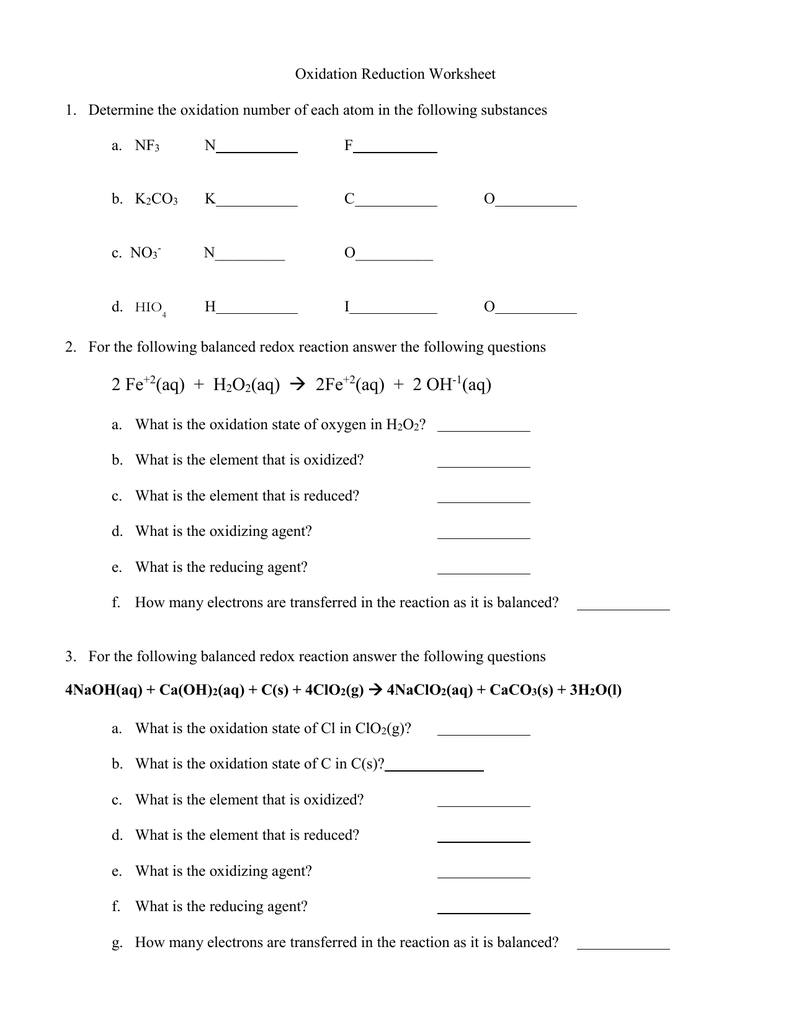
Write balanced equations for the following redox reactions a 2 NaBr Cl 2 2 NaCl Br 2 b Fe 2 O 3 3 CO 2 Fe 3 CO 2 in acidic solution c 5 CO I 2 O 5 5 CO 2 I 2 in basic solution a Cr OH 3 Br 2 CrO 42 Br in basic solution 10 OH 2 Cr OH 3 3 Br 2 2 CrO 42 8 H 2 O 6 Br b Answer a Sulphur in SO 3 will have the highest oxidation number Explanation To understand it better foremost we will calculate the oxidation number of SO 3 CO 2 AlCl 3 and CaS The oxidation number of sulphur in SO 3 is x 3 X 2 0 x 6 0 x 6 The oxidation number of carbon in CO 2 is
2 The oxidation number of a monatomic ion equals the charge on the ion 3 The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion 4 The oxidation number of fluorine in a compound is always 1 5 Oxygen has an oxidation number of 2 unless it is combined with F when Q1 Show the electron configuration for the transition metal cation using the box notation in the table Indicate if the complex is paramagnetic or not in the final column of the table Complete the oxidation number column of the table below by working out the oxidation number of each of the transition metal cations

6 Oxidation Numbers Worksheet Answers FabTemplatez
http://www.fabtemplatez.com/wp-content/uploads/2018/03/oxidation-numbers-worksheet-answers-87074-redox-balancing-oxidation-numbers-worksheet-answers-7201280.jpg

Oxidation Numbers Worksheet 2006 2024 Form Fill Out And Sign
https://www.signnow.com/preview/369/487/369487255/large.png
Oxidation Number Worksheet And Answers - Chemistry Chapter 7 Worksheets Chemical Formulas and Nomenclature page 1 WORKSHEET 1 Determination of oxidation number or valence number Rules to apply 1 a The net charges on all molecules is zero therefore the sum of the positive charges equals the sum of the negative charges 2 a