Le Chatelier S Principle Practice Worksheet Answers LE CHATELIER S PRINCIPLE CONTINUED Name 2Hl g 12 6 kcal Equilibrium Shift right 2 3 4 5 8 9 10 Stress Add H2 Add 12 Add HI Remove 1 12
Le hatelier s Principle Name Slot P a g e 1 Le Chatelier s Principle Worksheets 1 What is the effect on the total pressure inside a closed vessel if Le Ch telier s Principle Worksheet 1 What would happen to the position of the equilibrium when the following changes are made to the equilibrium system below CH4 g 2H2S g CS2 g 4H2 g a Decrease the concentration of dihydrogen sulfide hydrosulfuric acid Equilibrium will shift to favor reactants
Le Chatelier S Principle Practice Worksheet Answers

Le Chatelier S Principle Practice Worksheet Answers
https://i1.wp.com/s3.studylib.net/store/data/007513484_2-79f3d267309235356cf35a5f0c320a55.png
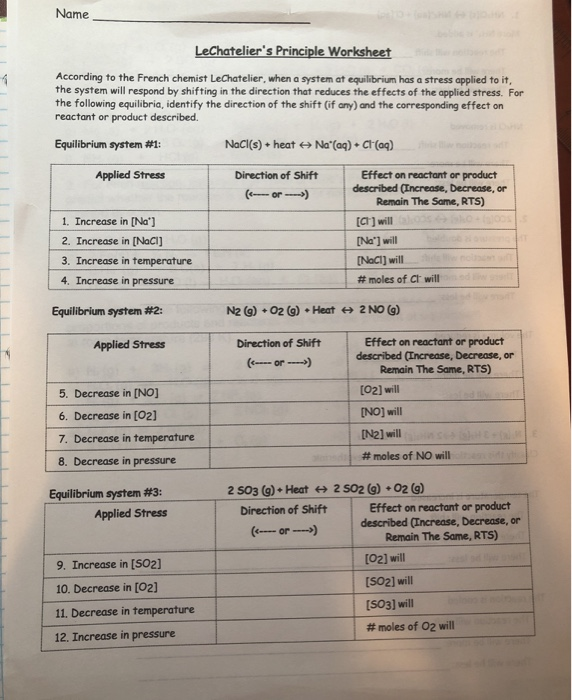
Le Chatelier s Principle Worksheet 2 Answers
https://media.cheggcdn.com/study/0b1/0b135d26-f047-4944-b05e-c15a2633a247/image.png
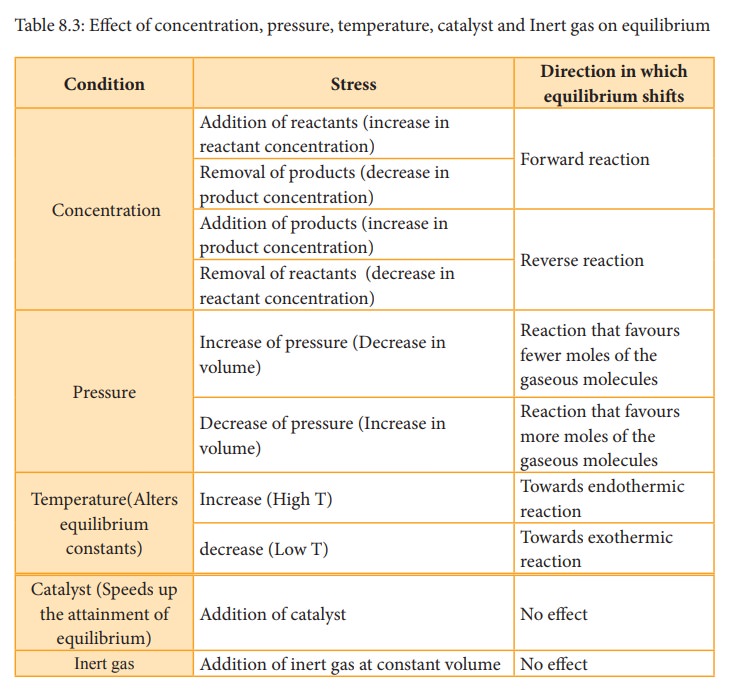
Basic Definition Of Le Chatelier s Principle CHEMISTRY COMMUNITY
https://img.brainkart.com/imagebk37/N8qe47J.jpg
Worksheet Le Chatelier s Principle Name CHEMISTRY A Study of Matter 2004 GPB 12 11 If a system at equilibrium is subjected to a the equilibrium is Lab Worksheet for Chemical Equilibrium and Le Chatelier s Principle General Instructions Complete Part A Part B Steps 1a 1e skip 1f and Steps 2a 2e skip 2f 2i Follow the procedure in the lab manual and record your data on this worksheet As your laboratory report turn in to your TA this worksheet along with the appropriate pages from
Le Ch telier s principle Google Classroom 2 SO A 2 g O A 2 g 2 SO A 3 g After the system above reaches equilibrium additional O A 2 g is injected into the reaction flask at a time t 1 while the temperature is held constant Using Le Chatelier s principle determine the change in the amount of ZnCl 2 when OH is added to the solution to produce the complex ion Zn OH 4 2 13 PRACTICE PROBLEM Methane can be produced from carbon monoxide via catalytic hydrogenation according to the following reaction CO g 3 H 2 g CH 4 g H 2 O g H
More picture related to Le Chatelier S Principle Practice Worksheet Answers

Le Chatelier s Principle Grade 12 Practice YouTube
https://i.ytimg.com/vi/8MeJtBY37a8/maxresdefault.jpg
Le Ch telier s Principle And The Equilibrium Constant Study Guide
https://images.ctfassets.net/4yflszkpcwkt/35U4eWcIqfsRew0w3wqG8b/99914dde0dc50fc9d01ab769770b1fa4/005792371_1-f77cf01f82d11e050eec1a305649e9ef.png

Le Chatelier s Principle Chemistry Practice Worksheet CHEM 1060 U
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/451b8f47215bad36373a6ab502164f13/thumb_1200_1553.png
Le Chatelier s Principle Worksheet 1 1 For the reaction below which change would cause the equilibrium to shift to the right Decrease the concentration of dihydrogen sulfide Increase the pressure on the system Increase the temperature of the system Increase the concentration of carbon disulfide Decrease the concentration of methane 2 Le Chatelier s Principle Worksheet 2 1 In the following reaction will the H 2 increase or decrease when equilibrium is reestablished after these stresses
4 Consider the following equilibrium process for the commercial production of hydrogen CO g H 2 O g CO 2 g H 2 g H 42 kJ mol Predict the direction of the shift in equilibrium when a the temperature is raised b more CO gas is added to the reaction mixture Dougherty Valley HS Chemistry Equilibrium Le Chatelier s Principle and Keq Practice Name Period Seat Directions Complete the following chart by choosing from the following options Equilibrium Shift left right no change Temp increase decrease no change Keq no yes N 2 g
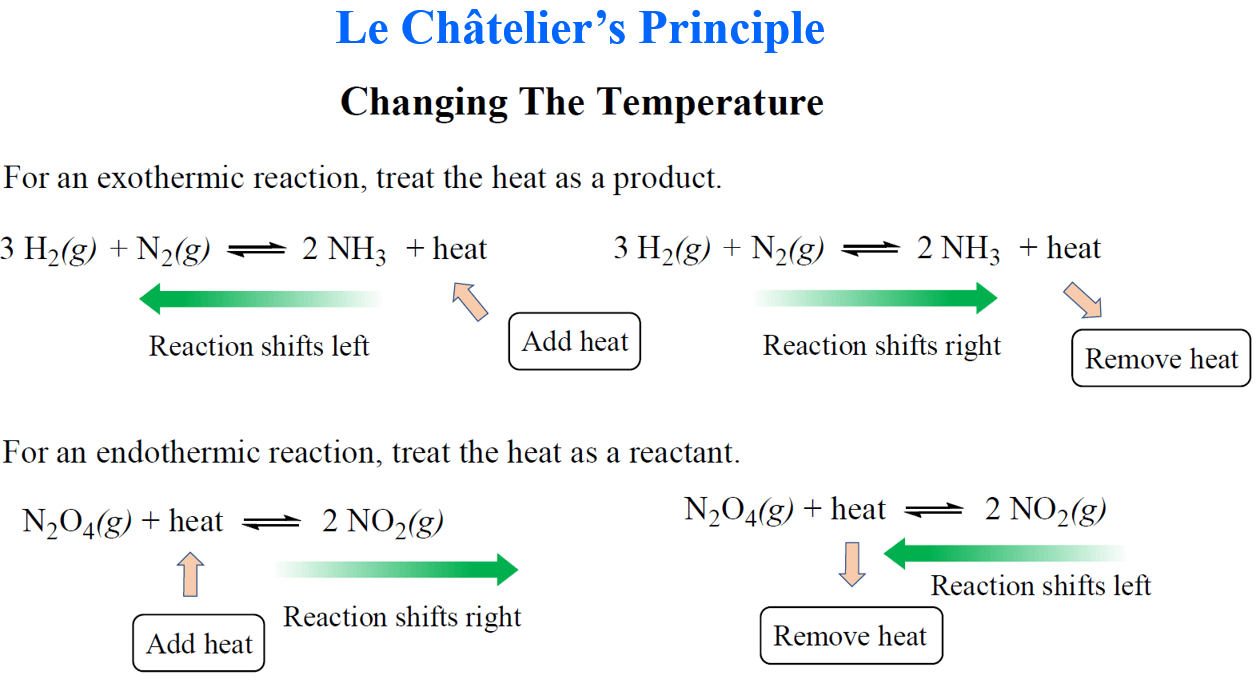
Le Chateliers Principle Chemistry Steps
https://general.chemistrysteps.com/wp-content/uploads/2022/05/Le-Chateliers-Principle-Changing-Temperature-at-Equilibrium.png

Le Chatelier s Principle Worksheet For 10th Higher Ed Lesson Planet
https://content.lessonplanet.com/resources/thumbnails/44702/original/bwluav9tywdpy2symde3mdmymi0xnjuwmi1rmtv1mxauanbn.jpg?1490214019
Le Chatelier S Principle Practice Worksheet Answers - Using Le Chatelier s principle determine the change in the amount of ZnCl 2 when OH is added to the solution to produce the complex ion Zn OH 4 2 13 PRACTICE PROBLEM Methane can be produced from carbon monoxide via catalytic hydrogenation according to the following reaction CO g 3 H 2 g CH 4 g H 2 O g H