Electron Configuration Orbital Notation Worksheet A typical electron configuration consists of numbers letters and superscripts with the following format 1 A number indicates the energy level The number is called the principal quantum number 2 A letter indicates the type of orbital s p d f 3 A superscript indicates the number of electrons in the orbital Example ls
Draw the orbital diagrams for the following IONS This will be the same orbital diagrams as a neutral atom except you ve added or subtracted some arrows to represent the electrons that were added or subtracted Write the ground state electron configuration of the following neutral elements in orbital notation orbital notation with arrows and in short hand noble gas notation
Electron Configuration Orbital Notation Worksheet

Electron Configuration Orbital Notation Worksheet
https://www.unmisravle.com/wp-content/uploads/2018/04/electron_configuration_worksheet__configurations__practice_0.png

Orbital Notation Worksheets
https://www.housview.com/wp-content/uploads/2018/04/writing_electron_configuration_worksheet_6.jpg
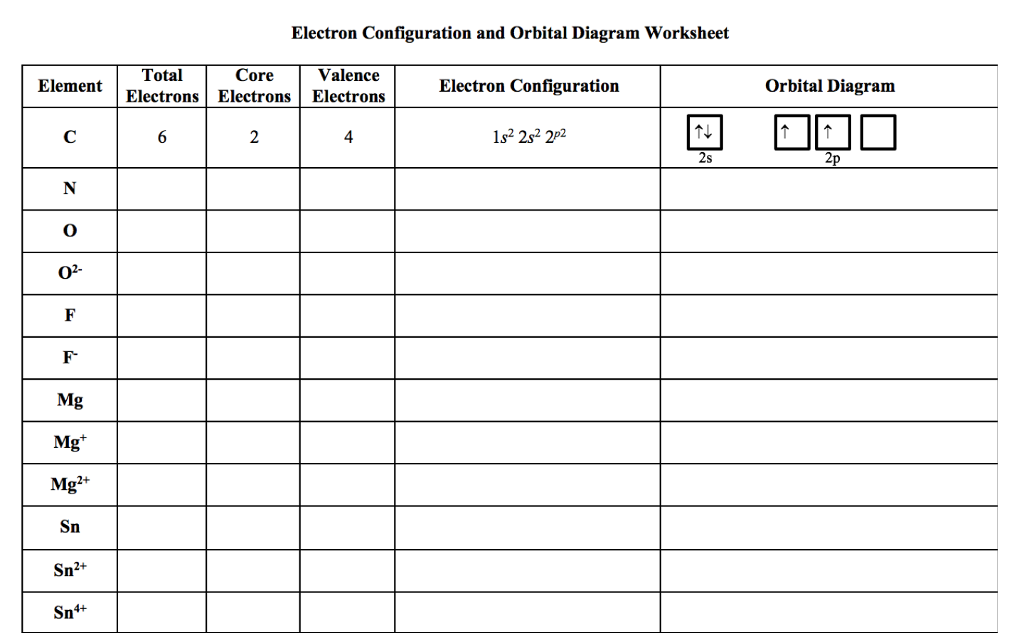
Solved Electron Configuration And Orbital Diagram Worksheet Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/3e8/3e8f9e19-d38e-4214-bb0f-a709675130f7/phpUUN6gx.png
Use the patterns within the periodic table to write the Noble Gas electron configurations for the following elements 7 8 9 10 Each orbital diagram below violates one of the three rules of electron configuration An Electron Configuration is a shorthand notation that is used to describe the locations of all of the electrons in an atom The electron configuration for a helium atom is He 1s2 This means that there are 2 electrons in an s orbital The s orbital is located in
An electron configuration is a method of indicating the arrangement of electrons about a nucleus A typical electron configuration consists of numbers letters and superscripts with the following format 1 A number indicates the energy level The number is called the principal quantum number 2 A letter indicates the type of orbital s p Where are the Electrons Write the full electron configuration short hand electron configuration and fill in the orbital diagrams for the following elements
More picture related to Electron Configuration Orbital Notation Worksheet

Orbital Notation Worksheets
https://www.housview.com/wp-content/uploads/2018/04/pictures_electron_configuration_review_worksheet_4.gif

What Is The Shape Of F orbital Example
https://useruploads.socratic.org/2MKFIOEQDOHTSCWyWNyY_ORBITALS_-_4forbitals.png

Electron Configuration Practice Worksheet Acetolease
https://acetolease.weebly.com/uploads/1/3/4/2/134256533/182221156_orig.jpg
Nucleus A typical electron configuration consists of numbers letters and superscripts with the following format 1 A number indicates the energy level The number is called the principal quantum number 2 A letter indicates the type of orbital s p d f 3 A superscript indicates the number of electrons in the orbital Example ls2 These worksheets test students knowledge of electron configuration and orbital diagrams Students must have prior knowledge of Pauli Exclusion Principle Aufbau Principle and Hund s Rule to understand how electrons fill up the various atomic orbitals
How to write an electron configuration A Determine the total number of electrons to be represented B Use the Aufbau principle to fill the orbitals with electrons for elements 1 23 Energy levels sublevels of orbitals and spin of electrons are all provided and shown with orbital notations PART A ELECTRON CONFIGURATIONS AND ORBITAL NOTATIONS Use the patterns within the periodic table to write the orbital notation for the following atoms
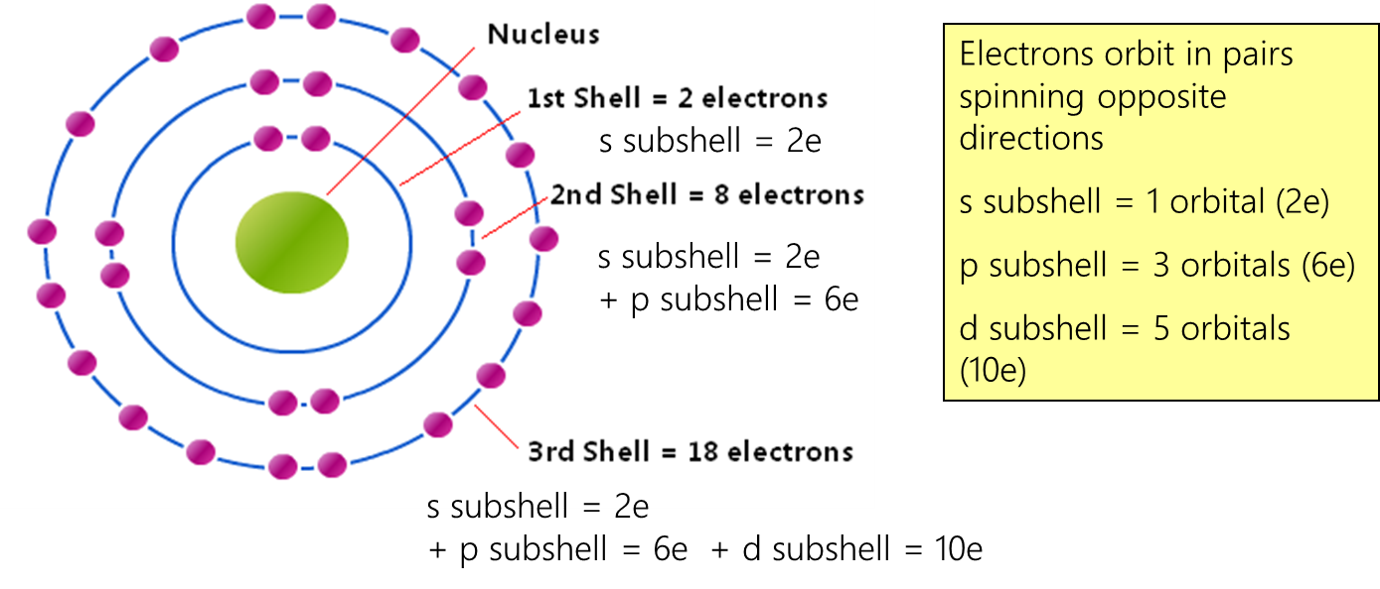
1 Electron Configuration
http://gzscienceclassonline.weebly.com/uploads/1/1/3/6/11360172/electron-orbitals_orig.png

Question Video Identifying The Correct Sequence Of Orbital Diagrams To
https://media.nagwa.com/840104164268/en/thumbnail_l.jpeg
Electron Configuration Orbital Notation Worksheet - Electron Configuration Notations These notations provide a way to describe the location and spin of every electron in an atom Orbital Notation This involves drawing each orbital as a box and representing electrons as arrows The direction of the arrow up or