Specific Heat And Heat Capacity Worksheet Answers 3 Calculate the energy of combustion for one mole of butane if burning a 0 367 g sample of butane C 4 H 10 has increased the temperature of a bomb calorimeter by 7 73 C The heat capacity of the bomb calorimeter is 2 36 kJ C answer This content is available to registered users only
Problem 9 A piece of metal of unknown specific heat weighing 25 g and temperature 90 C is dropped into 150 g of water at 10 C They finally reach thermal equilibrium at a temperature of 24 C Calculate the unknown specific heat capacity Specific heat of water is 1 0 cal g C Specific Heat Worksheet Name in ink C q mAT where q heat energy m mass and T temperature Remember AT Tfinal Tinitial Show all work and proper units Answers are provided at the end of the worksheet without units 1 A 15 75 g piece of iron sorbs 1086 75 joules of heat energy and its temperature changes from 25 0 1750C
Specific Heat And Heat Capacity Worksheet Answers

Specific Heat And Heat Capacity Worksheet Answers
https://storage.googleapis.com/worksheetzone/image/633cec6dca1047184feb5099/specific-heat-worksheet-answers-1-w1000-h1294-preview-0.png
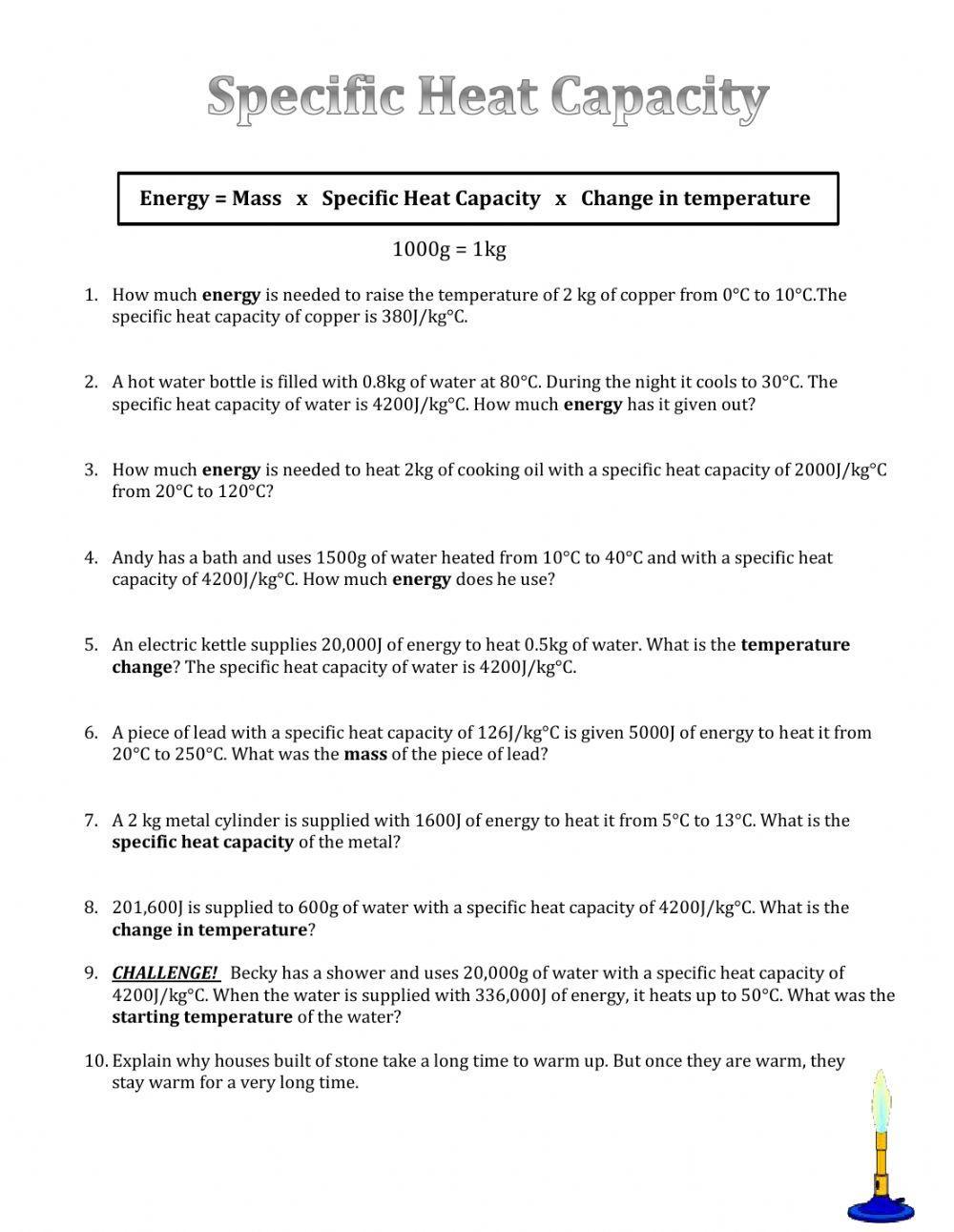
Specific Heat Capacity Interactive Worksheet Live Worksheets
https://www.liveworksheets.com/sites/default/files/styles/worksheet/public/def_files/2021/1/19/101190904301163121/101190904301163121002.jpg?itok=9tUszniz

Calculating Specific Heat Worksheet
https://db-excel.com/wp-content/uploads/2019/09/specific-heat-capacity-worksheet-key-engineering-studocu-2.png
In this problem we re given the mass of the object the specific heat capacity and the change in temperature which allows us to calculate the heat energy Problem 15 The temperature of a sample of iron with a mass of 10 0 g changed from 50 4 C to 25 0 C with the release of 47 Joules of heat Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24 5oC to 26 4oC How much heat did this sample absorb c for water 4 18 J goC ans 2 66 kJ 2 How much heat in kilojoules has to be removed from 225g of water to lower its temperature from 25 0oC to 10 0oC ans 14 1 kJ
The specific heat capacity is intensive and does not depend on the quantity but the heat capacity is extensive so two grams of liquid water have twice the heat capacitance of 1 gram but the specific heat capacity the heat capacity per gram is the same 4 184 J g K Table 1 1 Specific Heats of Common Substances at 25 C and 1 bar Worksheet Calculations involving Specific Heat 1 For q m c T identify each variables by name the units associated with it 2 Heat is not the same as temperature yet they are related Explain how they differ from each other a Perform calculations using q m c T b
More picture related to Specific Heat And Heat Capacity Worksheet Answers

Specific Heat And Heat Capacity Worksheet
https://s3.studylib.net/store/data/008472477_1-657c0256926328f7dc64ac4b7dee5831-768x994.png

Heat Capacity And Specific Heat Worksheet For 11th 12th Grade
https://content.lessonplanet.com/resources/thumbnails/276134/original/odu4ndaylmpwzw.jpg?1414473680

Specific Heat Worksheet Answer Key
https://static.docsity.com/documents_first_pages/2021/04/20/8383defce09a810692f71a4f70fe05f4.png
Specific heat is closely related to the concept of heat capacity Heat capacity is the amount of heat necessary to change the temperature of a substance by 1 00 C C In equation form heat capacity C is C mc C m c where m is mass and c is specific heat Note that heat capacity is the same as specific heat but without any dependence Specific heat capacity c can be experimentally determined by measuring the temperature change T Tf Ti in C that a known mass m undergoes when it loses or gains a quantity of heat q so it follows that q m c T T Recall that the density of pure water 1 00 g mL 1 so that for water 100 g 100 cm3 No Question
Latent heat and Specific heat capacity questions 1 How much water at 50 C is needed to just melt 2 2 kg of ice at 0 C 2 How much water at 32 C is needed to just melt 1 5 kg of ice at 10 C 3 How much steam at 100 is needed to just melt 5 kg of ice at 15 C 4 A copper cup holds some cold water at 4 C Assume that the coffee has the same density and specific heat as water Answer The temperature of the coffee will drop 1 degree the temperature increases by 1 48 C If the heat capacity of the calorimeter is 21 6 kJ C determine the heat produced by combustion of a ton of coal 2000 pounds Remember 1 kg 2 2 pounds Answer 2 91

Specific Heat And Heat Capacity Worksheet DocsLib
https://data.docslib.org/img/9787957/specific-heat-and-heat-capacity-worksheet.jpg
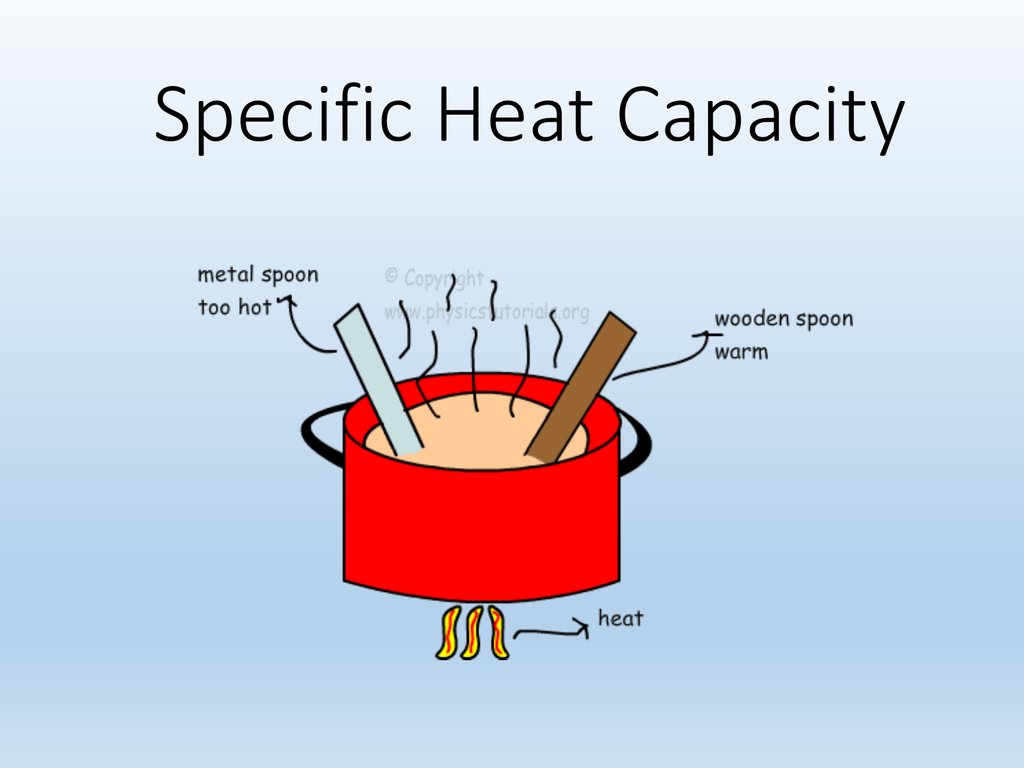
1 3 Specific Heat Capacity
https://s2.studylib.net/store/data/018037878_1-5dc8c711621f05e80a2a3975e8530b10.png
Specific Heat And Heat Capacity Worksheet Answers - Worksheet Calculations involving Specific Heat 1 For q m c T identify each variables by name the units associated with it 2 Heat is not the same as temperature yet they are related Explain how they differ from each other a Perform calculations using q m c T b