Specific Heat Worksheet Answers Specific Heat Problems with Answers Several specific heat practice problems with solutions are provided for high school students Each problem discusses the definition and formula of specific heat Specific heat capacity is defined as the amount of heat energy Q required to raise the temperature of a unit mass one kilogram or one gram of
3 Calculate the energy of combustion for one mole of butane if burning a 0 367 g sample of butane C 4 H 10 has increased the temperature of a bomb calorimeter by 7 73 C The heat capacity of the bomb calorimeter is 2 36 kJ C answer This content is available to registered users only Specific Heat and Heat Capacity Worksheet DIRECTIONS Use q m Cp T to solve the following problems Show all work and units Ex How many joules of heat are needed to raise the temperature of 10 0 g of aluminum from 22 C to 55 C if the specific heat of aluminum is 0 90 J g C 1 The temperature of 335 g of water changed from 24
Specific Heat Worksheet Answers

Specific Heat Worksheet Answers
https://content.lessonplanet.com/resources/thumbnails/276134/original/odu4ndaylmpwzw.jpg?1414473680

Heat Transfer Specific Heat Problems Worksheet
https://s3.studylib.net/store/data/008722519_1-f32a1633ccad4f9d438e0e7a0ce7a9f8.png

50 Specific Heat Worksheet Answers
https://chessmuseum.org/wp-content/uploads/2019/10/specific-heat-worksheet-answers-new-specific-heat-worksheet-answers-of-specific-heat-worksheet-answers-1.jpg
Answers mc T where Q heat energy m mass and T change in temp Remember T Tfinal Tinitial Show all work and proper units A 15 75 g piece of iron absorbs 1086 75 joules of heat energy and its temperature changes from 25 C to 175 C Calculate the specific heat capacity of iron How many joules of heat are needed 50 g of gold with a specific heat of 0 129 is heated to 115 0C the gold cools until the final q m x c x T q metal J Problems Write the Formula set up problem and answer in correct units 1 Ethanol which has a specific heat of 2 44 J gx0C The temperature of 34 4 g of ethanol increases from 25 0C to 78 8 0C
The specific heat capacity is intensive and does not depend on the quantity but the heat capacity is extensive so two grams of liquid water have twice the heat capacitance of 1 gram but the specific heat capacity the heat capacity per gram is the same 4 184 J g K Table 1 1 Specific Heats of Common Substances at 25 C and 1 bar Worksheet Calculations involving Specific Heat 1 For q m c T identify each variables by name the units associated with it 2 Heat is not the same as temperature yet they are related Explain how they differ from each other a Perform calculations using q m c T b
More picture related to Specific Heat Worksheet Answers

20T Specific Heat Worksheet YouTube
https://i.ytimg.com/vi/xqKcPGkfb-I/maxresdefault.jpg

Specific Heat Worksheet Answers
https://briefencounters.ca/wp-content/uploads/2018/11/specific-heat-worksheet-answers-as-well-as-calorimetry-and-specific-heat-capacity-worksheet-of-specific-heat-worksheet-answers.png
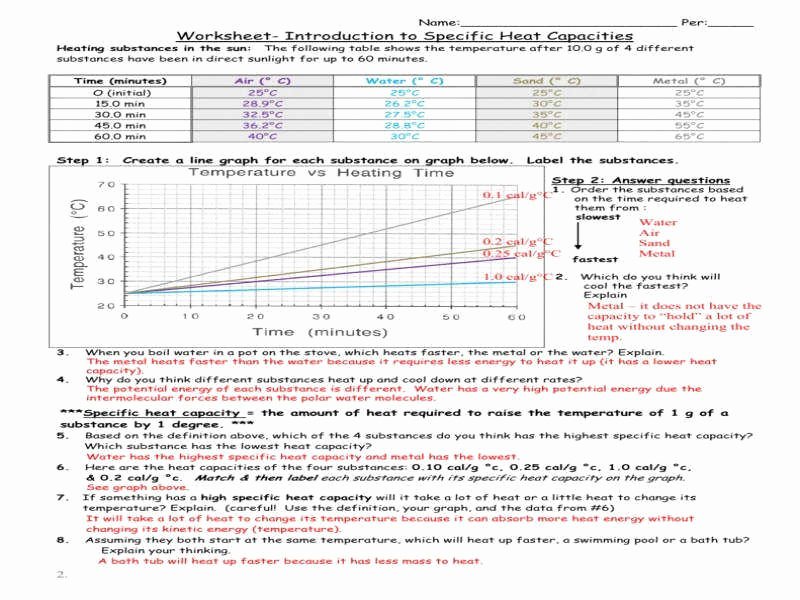
50 Specific Heat Worksheet Answer Key
https://chessmuseum.org/wp-content/uploads/2019/10/specific-heat-worksheet-answer-key-inspirational-specific-heat-worksheet-answers-of-specific-heat-worksheet-answer-key.jpg
Ch 16 Specific Heat Problems q mc T Specific heat of water 4 J g C or 1 cal g C 1 Convert the following 16 1 specific heat practice part 1 answer key Subject Chemistry 999 Documents Students shared 2356 documents in this course Level Ions Worksheet Chemistry 100 9 More from NHS HA More from NHS HA 999 The unit for specific heat is Joule g oC or Cal g C Specific heats are used to find the quantity of heat q that flows into or out of a system the equation for quantity of heat or enthalpy is q mass Temp change Cp sometimes written q m TCp Use this equation and the table of specific heats to solve the following problems 1
Specific heat capacity c can be experimentally determined by measuring the temperature change T Tf Ti in C that a known mass m undergoes when it loses or gains a quantity of heat q so it follows that q m c T T Recall that the density of pure water 1 00 g mL 1 so that for water 100 g 100 cm3 No Question Suitable for GCSE and A Level Physics 50 assorted questions answers included Resource includes Worksheet 50 mixed questions including 10 challenging questions Worksheet with answer space Answer sheet Worked solutions Solutions only Table of specific heat capacity values
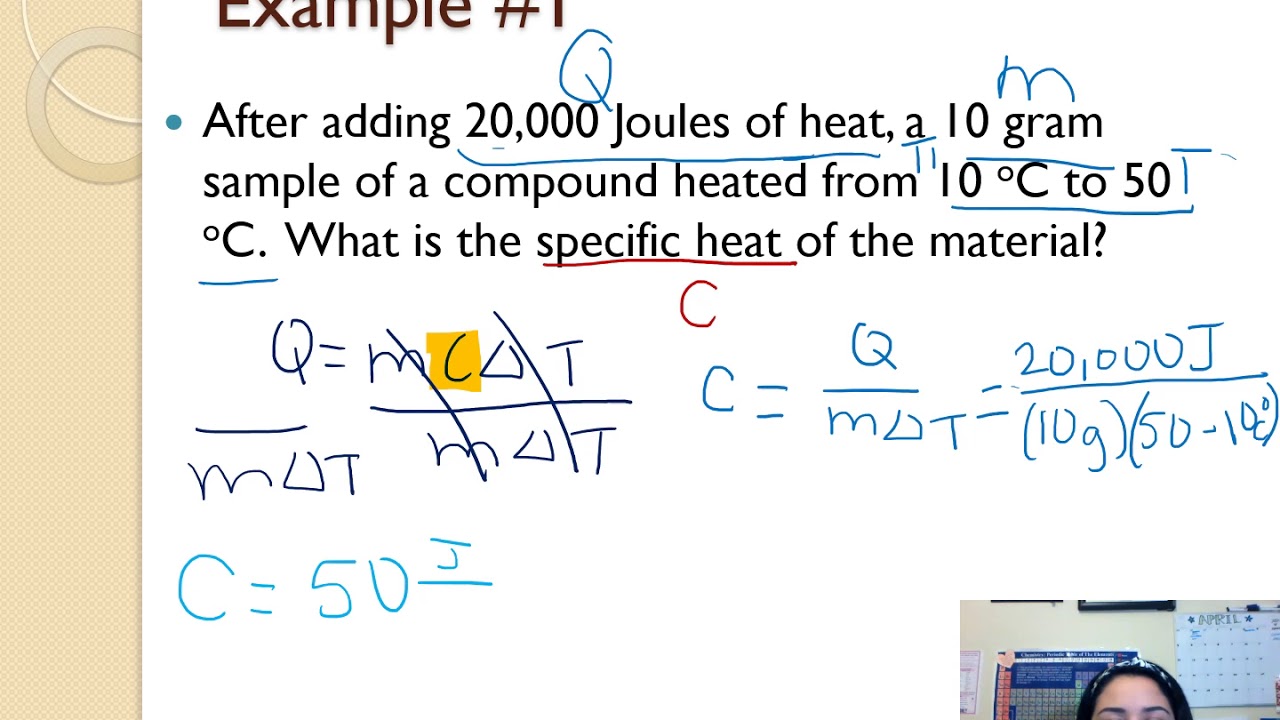
Specific Heat Calculations Worksheet Answers Printable Word Searches
https://i.ytimg.com/vi/YD38Ie9aLhs/maxresdefault.jpg

Specific Heat Worksheet Answer Key
https://briefencounters.ca/wp-content/uploads/2018/11/specific-heat-worksheet-answer-key-along-with-human-population-worksheet-answers-fresh-human-population-growth-of-specific-heat-worksheet-answer-key.jpg
Specific Heat Worksheet Answers - 2024 The amount of heat required to raise the temperature of one gram of a substance by one Celsius degree is referred to as specific heat Specific heat is usually measured in calories or joules per gram per Celsius degree Water for example has a specific heat of 1 calorie or 4 186 joules per gram per Celsius degree