Bronsted Lowry Acids And Bases Worksheet Answers A lower pKa is associated with a larger Ka which signifies greater dissociation The large relative difference in acidity in this case can be most easily seen by gauging the relative basicities of the conjugate bases The weaker the base the stronger the corresponding conjugate acid
Created Date 5 15 2014 12 44 28 PM Conceptual Questions Acids Bases and Conjugates Miscellaneous 1 In the Br 248 nsted Lowry definition of acids and bases an acid a is a proton donor d breaks stable hydrogen bonds b is a proton acceptor e corrodes metals c forms stable hydrogen bonds 2 In the Br 248 nsted Lowry definition of acids and bases a base a
Bronsted Lowry Acids And Bases Worksheet Answers

Bronsted Lowry Acids And Bases Worksheet Answers
https://i.ytimg.com/vi/nEXrjHa9NHE/maxresdefault.jpg
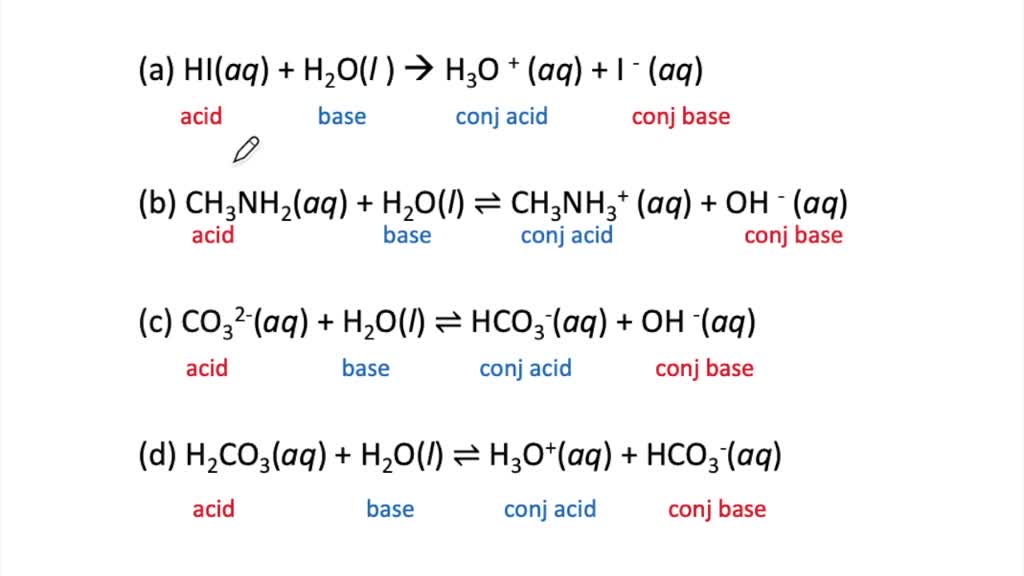
SOLVED The Bronsted Lowry Theory Exercise A Identify The Bronsted Acid
https://cdn.numerade.com/previews/c1d47fbf-6670-4d84-ac11-9d1cdf800ff6_large.jpg

Quiz Worksheet Bronsted Lowry And Lewis Definition Of Acids And
https://study.com/academy/practice/quiz-worksheet-bronsted-lowry-and-lewis-definition-of-acids-and-bases.jpg
Worksheet Bronsted Lowry Acids and Bases Name Period Date Identity the conjugation acid base pairs in the following reactions An acid donates a proton to become a conjugate base A base accepts a proton to form a conjugate acid 1 TASK 1 Bronsted Lowry acids amp bases Identify the Bronsted Lowry acid and base in each of the following reactions SECTION 2 pH of strong acids Number of protons released Monoprotic acid acid that releases one H ion per molecule e g HCl hydrochloric acid HNO3 nitric acid CH3COOH ethanoic acid Diprotic acid
6 2 The Bronsted Lowry Theory of Acids and Bases Worksheet 1 Fill in the chart below by providing simple definitions 2 Identify the hydrogen ion donor s amp acceptor s in each of the following reactions Apr 26 2021 nbsp 0183 32 Br 248 nsted Lowry acids bases pranshichauhan Member for 4 years 1 month Age 14 Level 11 Language English en ID 947733 26 04 2021 Country code IN Country India School subject Chemistry 1061818 Main content Acids and bases 2000752 From worksheet author AP chemistry Other contents identify acid and base Loading ad
More picture related to Bronsted Lowry Acids And Bases Worksheet Answers
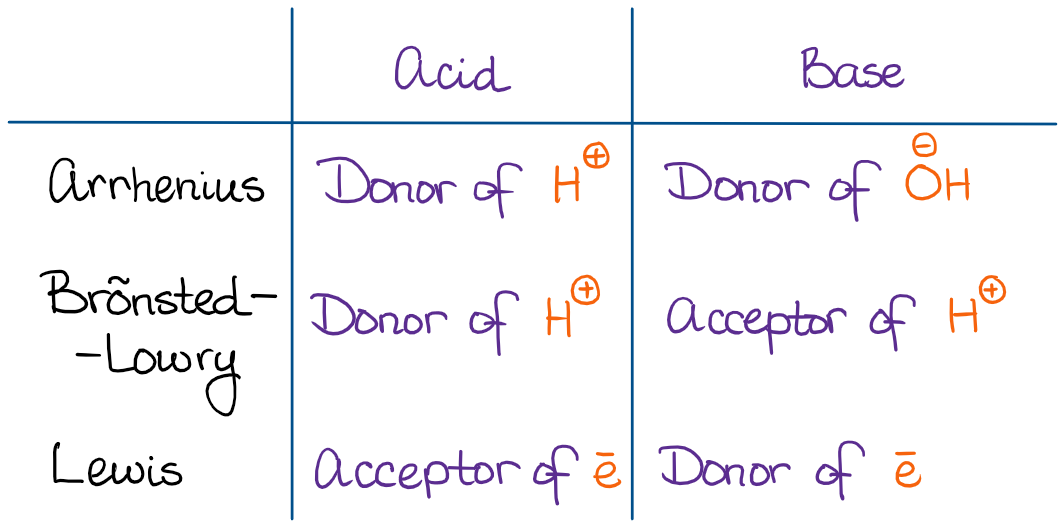
Introduction To Acids And Bases In Organic Chemistry Organic
https://www.organicchemistrytutor.com/wp-content/uploads/2019/09/AB-theories.png
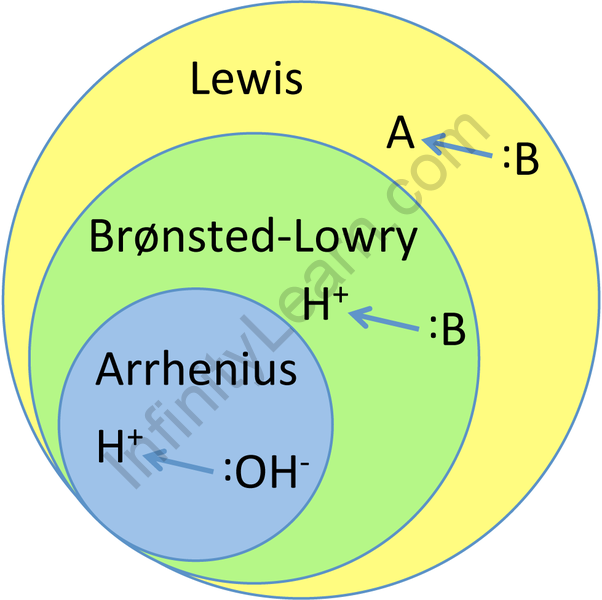
Bronsted Lowry Acid And Base Chemistry Infinity Learn By Sri Chaitanya
https://infinitylearn.com/surge/wp-content/uploads/2022/03/602px-Lewis-Bronsted-Arrhenius.png

Bronsted Lowry Acids And Bases Br nsted Lowry Reactions Example 1
https://i.ytimg.com/vi/tg_Qbast9go/maxresdefault.jpg
Lesson 1 Arrhenius Acids and Bases 1 Use Table K and Table L to help you identify the rules for determining whether a substance is an acid a base or a salt based on the formula Underline all the acids circle bases and box in salts purple Leave the covalent substances alone ACIDS and BASES Worksheet 1 Answers 1 Classify each of the following substances as an acid or a base according to the Arrenius Definition a ACID b BASE c BASE d ACID e BASE 2 Classify each of the following substances as an acid a base or amphoteric substance according to the Bronsted Lowry Definition a ACID b ACID c ACID d
[desc-10] [desc-11]

What Is The Br nsted Lowry Definition Of An Acid Of A Base Socratic
http://i.ytimg.com/vi/ZiokqP0aZ1E/maxresdefault.jpg

Bronsted Lowry Acid And Base Theory
https://sciencenotes.org/wp-content/uploads/2022/02/Bronsted-Lowry-Acid-and-Base-1024x683.png
Bronsted Lowry Acids And Bases Worksheet Answers - Worksheet Bronsted Lowry Acids and Bases Name Period Date Identity the conjugation acid base pairs in the following reactions An acid donates a proton to become a conjugate base A base accepts a proton to form a conjugate acid 1