Percent Yield Worksheet Answer Key What is the percent yield of I 2 if the actual grams produced is 39 78 grams of I 2 from 62 55 grams of NaI and excess of all the other reactants Answer 54 Actual Yield 39 78 g of I 2 Use 62 55 grams of NaI in Stoichiometry Equation to calculate the Theoretical Yield 62 55 1 1 I K H 150 1 I K H O 2
What is my percent yield 18 5 17 2 x 100 108 c Is the answer from problem b reasonable Explain No Any yield over 100 is a violation of the Law of conservation of mass d If I do this reaction with 15 grams of sodium sulfate and get a 65 0 yield how many grams of sodium phosphate will I make The percent yield of a reaction is the ratio of the actual yield to the theoretical yield multiplied by 100 to give a percentage percent yield actual yield g theoretical yield g 100 The method used to calculate the percent yield of a reaction is illustrated in Example 4 Example 4 Novocain
Percent Yield Worksheet Answer Key
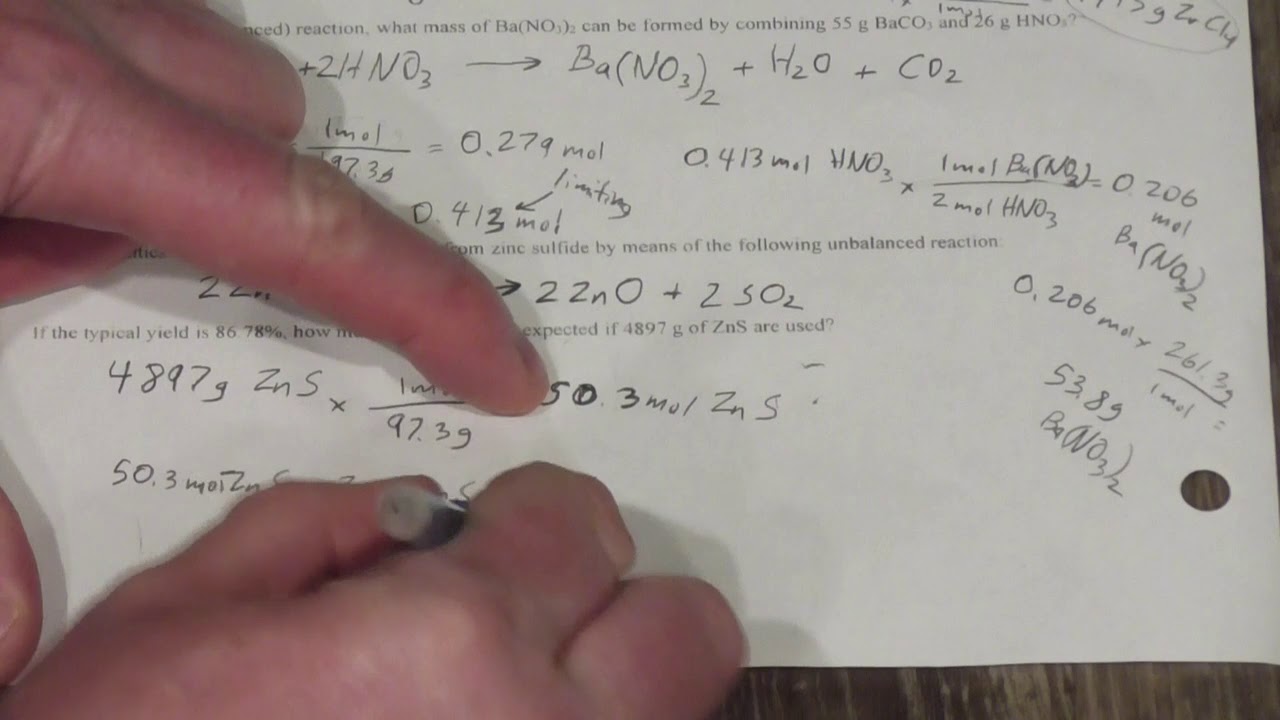
Percent Yield Worksheet Answer Key
https://i.ytimg.com/vi/xrdt7Xz8990/maxresdefault.jpg

Quiz Worksheet Calculating Reaction Yield And Percentage Db excel
https://db-excel.com/wp-content/uploads/2019/09/quiz-worksheet-calculating-reaction-yield-and-percentage-1.jpg

How To Calculate The Percent Yield Formula Modeladvisor
https://imgmidel.modeladvisor.com/1665036046310.jpg
Percent Yield Worksheet 1 Write a balanced equation for the reaction of tin IV phosphate with sodium carbonate to make tin IV carbonate and sodium phosphate 2 If 36 grams of tin IV phosphate is mixed with an excess of sodium carbonate how many grams of tin IV carbonate will form 3 If 29 8 grams of tin IV carbonate are actually Percent Yield Calculations Practice Problems 1 A reaction with a calculated yield of 9 23 g produced 7 89 g of product What is the percent yield for this reaction 2 5 96 g of ammonia 17 031 g mol react completely according to the following reaction 2 NH3 g C02 g What is the theoretical yield of urea CN20H4 60 056 for this reaction
My theoretical yield of beryllium chloride was 10 7 grams If my actual yield was 4 5 grams what was my percent yield Stoichiometry Percent Yield Worksheet Key 1 Write the equation for the reaction of iron III phosphate with sodium sulfate to make iron III sulfate and sodium phosphate 2 FePO4 3 Na2SO4 1 Fe2 SO4 3 2 Na3PO4 PROBLEM PageIndex 6 Freon 12 CCl 2 F 2 is prepared from CCl 4 by reaction with HF The other product of this reaction is HCl Outline the steps needed to determine the percent yield of a reaction that produces 12 5 g of CCl 2 F 2 from 32 9 g of CCl 4 Freon 12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long
More picture related to Percent Yield Worksheet Answer Key
Stoichiometry Worksheet 2 Percent Yield Answers Printable Word Searches
https://i2.wp.com/lh5.googleusercontent.com/proxy/jY_X0glEJFGV_Ujij0wpxnCXI7XRJkGy_Kv7Sp_N6j3RsE_RTo4Xo79DvDU6s6cPYiTh3hnJn5Nefz1690ZxjpgP8ubJ-LtmeiHhafn5g9d1e4QfmKq0gw3-bjsd4OrH=w1200-h630-p-k-no-nu
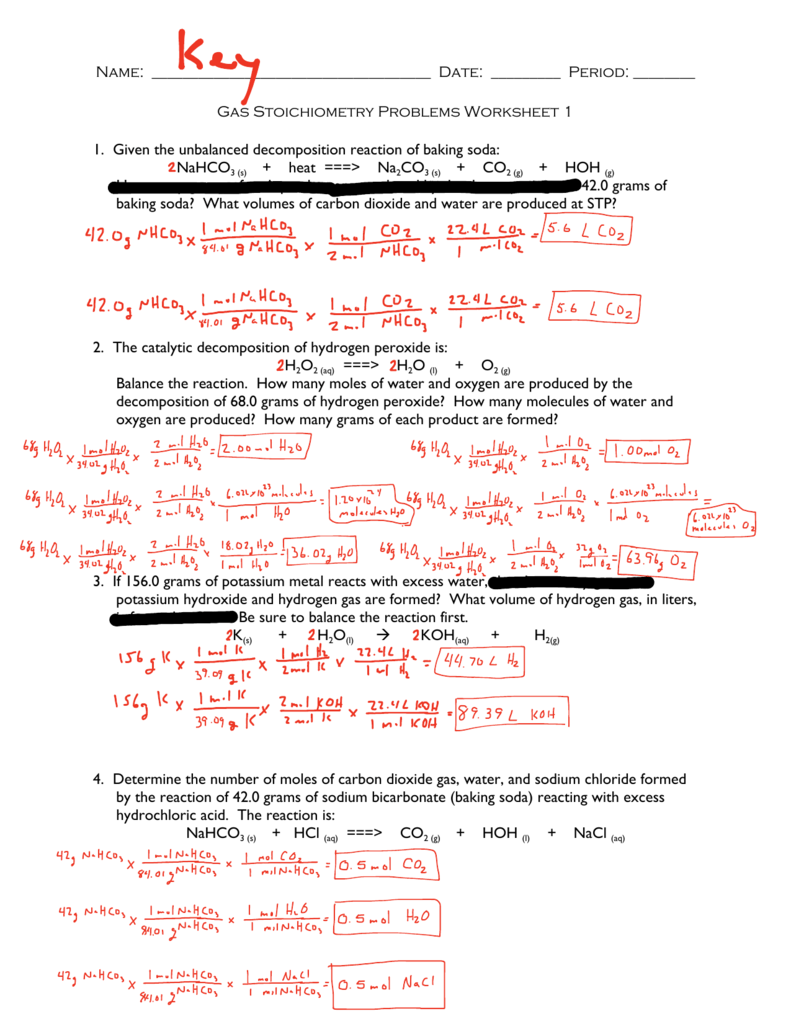
Gas Stoichiometry Worksheet Answer Key
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png
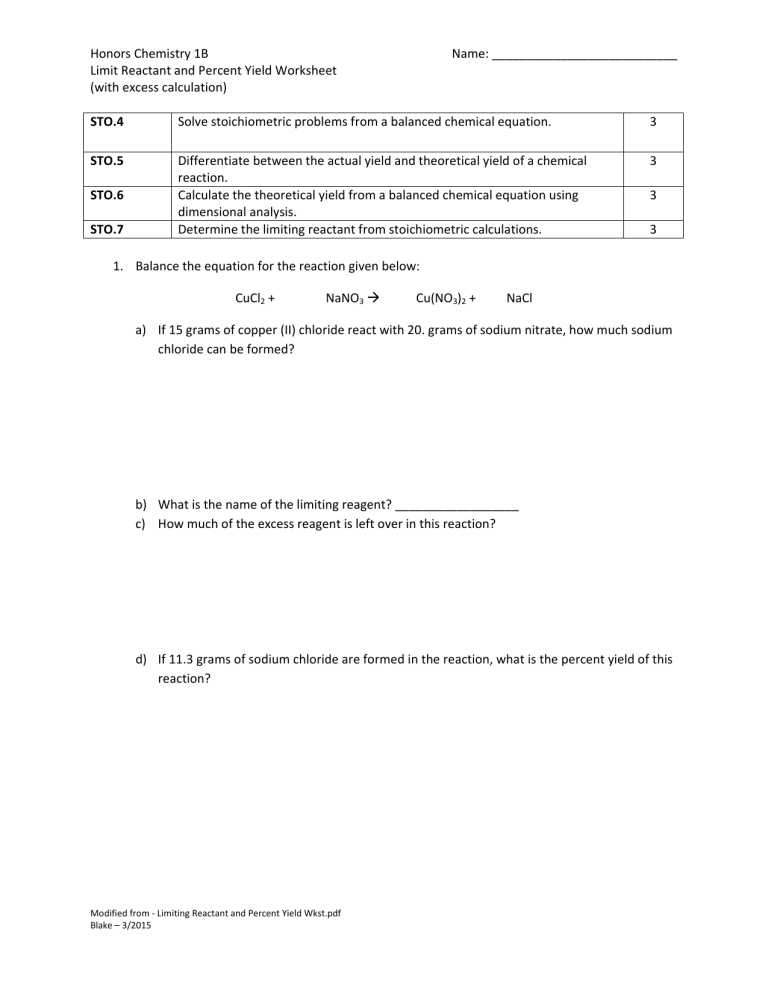
Limiting Reactant And Percent Yield Worksheet With Key
https://s3.studylib.net/store/data/025419473_1-3fe4a1364374e13854e48a2422b75d96-768x994.png
Correct answer 12 g x 100 Calculate the percent yield of a reaction that produced 5 78 g NH4 if its theoretical yield was 6 78 g 5 78 g NH4 is the actual yield Any time you see yield or produced assume that is the actual yield Percent yield 3 6 4 x 100 7 6 4 Percent yield 0 853 x 100 85 3 If 49 0 g of H 3 PO 4 is reacted with excess KOH determine the percent yield of K 3 PO 4 if you isolate 49 0 g of K 3 PO 4 3 Given the following equation Al 2 SO 3 3 6 NaOH g 3 Na 2 SO 3 2 Al OH 3 If you start with 389 4 g of Al 2 SO 3 3 and you isolate 212 4 g of Na 2 SO 3 what is your percent yield for this reaction 4 Given
O D O o o o D a o O z o z o o o o o o O cn o o X o o O o 03 D o O o o 00 o o D o o D o o o O O O o o Created Date 2 15 2012 7 16 58 AM Percent Yield The amount of product that may be produced by a reaction under specified conditions as calculated per the stoichiometry of an appropriate balanced chemical equation is called the theoretical yield of the reaction In practice the amount of product obtained is called the actual yield and it is often less than the theoretical yield for a number of reasons

Limiting Reactant And Percent Yield Worksheet Kidsworksheetfun
https://i.pinimg.com/originals/7d/30/4c/7d304c9fdd2f7b8ccfacfe75ba216b41.png

Percent Yield Worksheet Answer Key
https://briefencounters.ca/wp-content/uploads/2018/11/pemdas-worksheets-with-answers-along-with-percent-yield-worksheet-answers-worksheets-for-all-of-pemdas-worksheets-with-answers.jpg
Percent Yield Worksheet Answer Key - Modified from Limiting Reactant and Percent Yield Wkst pdf Blake 3 2015 2 Write the equation for the reaction of iron III phosphate with sodium sulfate to make iron III sulfate and sodium phosphate a If you perform this reaction with 25 grams of iron III phosphate and an excess of sodium sulfate how many grams of iron III