Stoichiometry Percent Yield Worksheet Answer Key Slaked lime Ca OH 2 is produced when water reacts with quick lime CaO If you start with 2 400 g of quick lime add excess water and produce 2 060 g of slaked lime what is the percent
Chapter 9 Percent Yield Test Practice Problems with Answer Key doc Shared Dec 20 2011 28 KB Download Chapter 9 Practice TEST pdf Shared Chemical Reactions of Copper and Practice worksheet on limiting reagents percent yield balancing equations and stoichiometry calculations in chemistry Ideal for high school students
Stoichiometry Percent Yield Worksheet Answer Key

Stoichiometry Percent Yield Worksheet Answer Key
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/637ac16783c73ade6498349091e2ac43/thumb_1200_1553.png

Limiting Reactant Worksheet Answers
https://i.ytimg.com/vi/s434qOiWyNA/maxresdefault.jpg

Unit 10 Worksheet 2 General Chemistry Stoichiometry And Percent Yield
https://s2.studylib.net/store/data/015557787_1-2d9070d8b296dc21d7c91e558d1a5f57.png
Percent Yield Calculations Practice Problems 1 A reaction with a 183 calculated yield of 9 23 g produced 7 89 g of product What is the percent yield for this reaction 2 5 96 g of ammonia Answer Key 1 Calculate the percent yield of a reaction that had a theoretical yield of 3 76 g and an actual yield of 1 45 g To answer this we just use the following equation Percent yield
What was the percent yield of the reaction Use the balanced equation to find out how many liters of sulfur dioxide are actually produced at STP if 1 5 x 1027 molecules of zinc sulfide are In this worksheet students calculate how much of the expected product was actually made by a reaction This is percent yield Essential concepts Actual yield percent yield theoretical yield
More picture related to Stoichiometry Percent Yield Worksheet Answer Key

REVIEW Stoichiometry Percent Yield Limiting Reagent
https://s2.studylib.net/store/data/009930367_1-67eebcc7459daa8b01b455e4c869b6cf-768x994.png
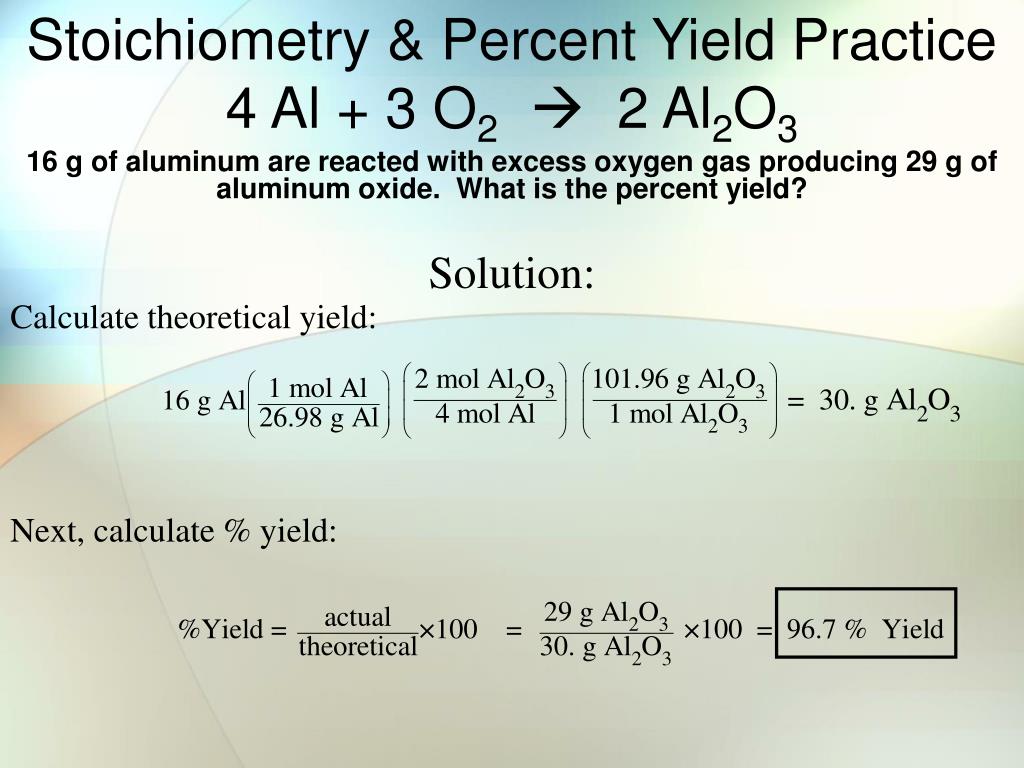
PPT Stoichiometry Percent Yield Practice 2 Al 6 HCl 2 AlCl 3 3
https://image3.slideserve.com/5527875/stoichiometry-percent-yield-practice-4-al-3-o-2-2-al-2-o-3-l.jpg
Stoichiometry Worksheet 2 Percent Yield Answers Printable Word Searches
https://i2.wp.com/lh5.googleusercontent.com/proxy/jY_X0glEJFGV_Ujij0wpxnCXI7XRJkGy_Kv7Sp_N6j3RsE_RTo4Xo79DvDU6s6cPYiTh3hnJn5Nefz1690ZxjpgP8ubJ-LtmeiHhafn5g9d1e4QfmKq0gw3-bjsd4OrH=w1200-h630-p-k-no-nu
1 Write a balanced equation for the reaction of tin IV phosphate with sodium carbonate to make tin IV carbonate and sodium phosphate 2 If 36 grams of tin IV phosphate is mixed with an An answer key is provided with the solutions to each problem This document is a stoichiometry worksheet containing 10 chemistry problems involving mole mole calculations determining
If 35 0 grams of bromine are reacted and 27 9 grams of phosphorous tribromide are formed what is the percent yield 2 Silver Nitrate reacts with Magnesium Chloride to produce Silver Ll iting Reagent amp Percent Yield Practice Worksheet 1 When copper Il chloride reacts with sodium nitrate copper Il nitrate and sodium chloride are formed a Write the balanced
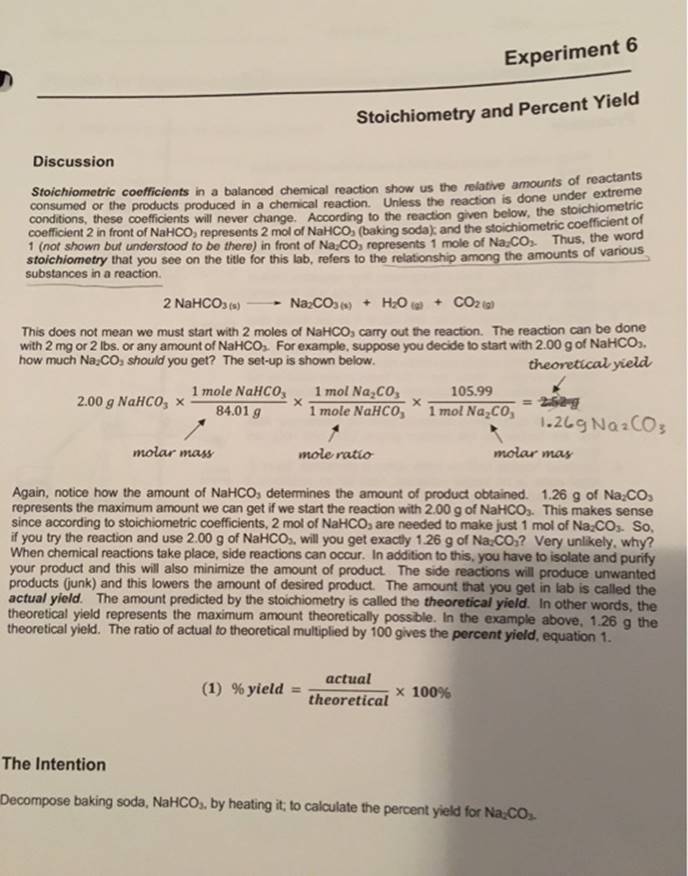
Get Answer Experiment 6 Stoichiometry And Percent Yield Discussion
https://files.transtutors.com/book/qimg/a2b0e6c0-c4a9-4ae0-91b3-dc9dae19944a.png
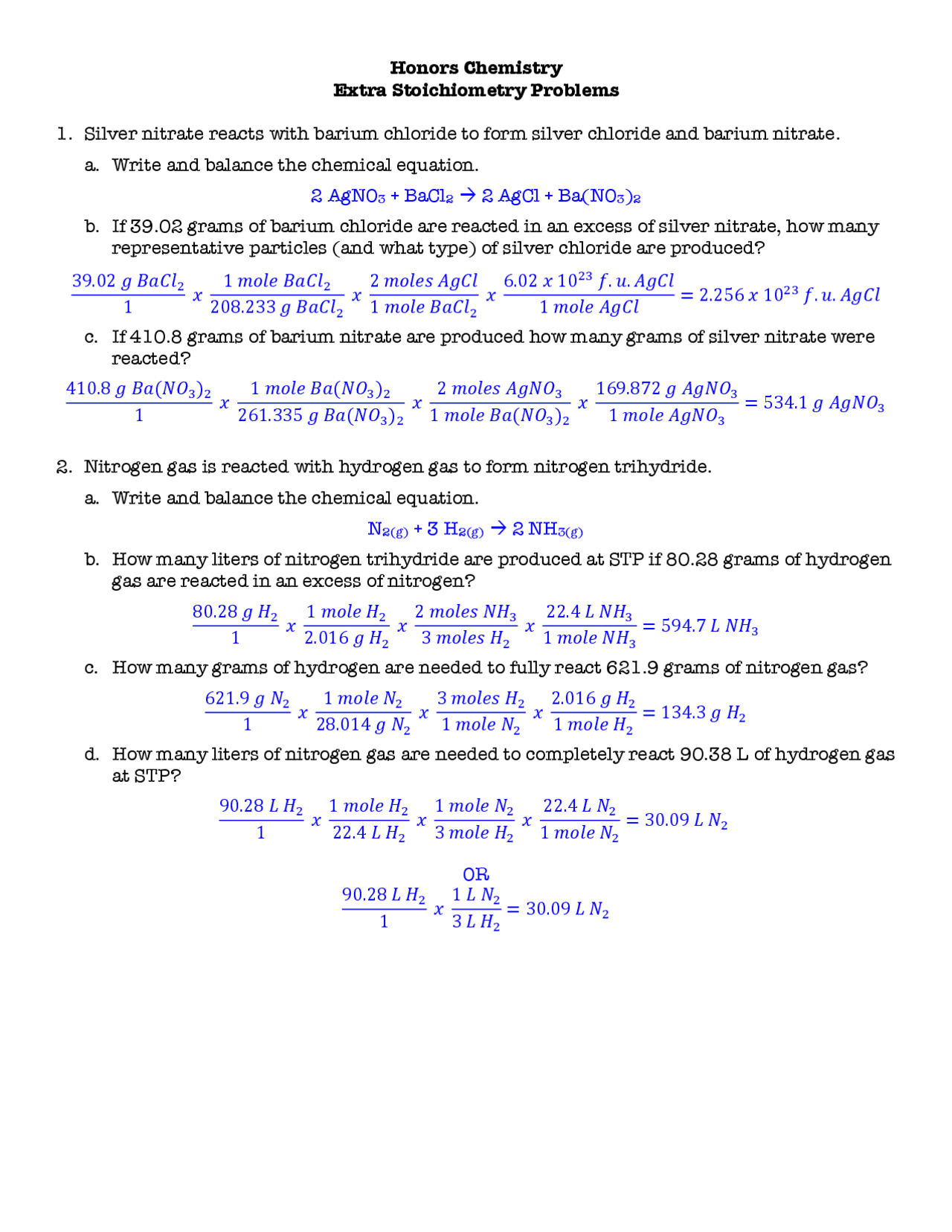
Stoichiometry Problems Worksheet Answers
https://static.docsity.com/documents_first_pages/2021/04/20/374813fc83f05cecfbdbe5809326f2b0.png
Stoichiometry Percent Yield Worksheet Answer Key - If you have data for the percent composition of a compound element by element do you need to know the size of the sample in order to figure out the empirical formula Why or why not No