12 3 Limiting Reagent And Percent Yield Worksheet Answer Key 12 3 Limiting reagent and percent yield Flashcards Learn Test Match Flashcards Learn Test Match Created by Alaysia Lane Terms in this set 5 Actual Yield Verified answer CHEMISTRY Show how phenylalanine can be prepared by reductive amination of an ketocarboxylic acid Verified answer
3 PO 4 2 Fe 2 CO 3 3 Answer the questions at the top of this sheet assuming we start with 100 grams of calcium carbonate and 45 grams of iron II phosphate iron III phosphate is limiting 46 3 grams of calcium phosphate 43 8 grams of iron III carbonate 54 0 grams of calcium carbonate 3 Write the balanced equation for the reaction The percent yield of a reaction is the ratio of the actual yield to the theoretical yield multiplied by 100 to give a percentage percent yield actual yield g theoretical yield g 100 The method used to calculate the percent yield of a reaction is illustrated in Example 4 Example 4 Novocain
12 3 Limiting Reagent And Percent Yield Worksheet Answer Key

12 3 Limiting Reagent And Percent Yield Worksheet Answer Key
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/2aa01efddac798a91d01c58231d6c279/thumb_1200_1553.png

How To Calculate The Percent Yield Formula Modeladvisor
https://imgmidel.modeladvisor.com/1665036046310.jpg

Percent Yield Worksheets
https://i2.wp.com/www.worksheeto.com/postpic/2015/10/percent-yield-worksheet-answers_224046.png
Step 3 Calculate the theoretical yield Our final step is to determine the theoretical yield of AlCl 3 in the reaction Remember that the theoretical yield is the amount of product that is produced when the limiting reactant is fully consumed In this case the limiting reactant is Cl A 2 so the maximum amount of AlCl 3 that can be formed is Find the limiting reagent 54g Al x 1 mole Al 27g Al 2 mole Al 319g CuSO4 x I mole CuSO4 159 5g CuSO4 2 mole CuSO4 limiting reagent 2 mole CuSO4 x 3 mole Cu 3 mole CuSO4 x 63 5g Cu 1 mole Cu 127g Cu Multiply the founded limiting reagent with moles of Copper 3 over moles of CuSO4 3 from the equation to cancel out moles of CuSO4
12 3 1 Identify the limiting reagent in a reaction 12 3 2 Calculate theoretical yield actual yield or percent yield given appropriate information Guide for Reading Build Vocabulary LINCS Have students use the LINCS strategy for the terms theoretical yield actual yield and percent yield Students should L ist the parts of a term they know I If you start with 14 82 g of Ca OH 2 C a O H 2 and 16 35 g of H2SO4 H 2 S O 4 a determine the limiting reagent b determine the number of moles of H2O H 2 O produced c determine the number of grams of CaSO4 C a S O 4 produced d determine the number of grams of excess reagent left 1 make sure the equation is balanced
More picture related to 12 3 Limiting Reagent And Percent Yield Worksheet Answer Key

Limiting Reagent Worksheet Answers Db excel
https://db-excel.com/wp-content/uploads/2019/09/limiting-reagent-worksheet-answers-4.jpg

Limiting Reactant And Yield Worksheet
https://s3.studylib.net/store/data/008633154_1-36978410235575486330534459741fcb-768x994.png
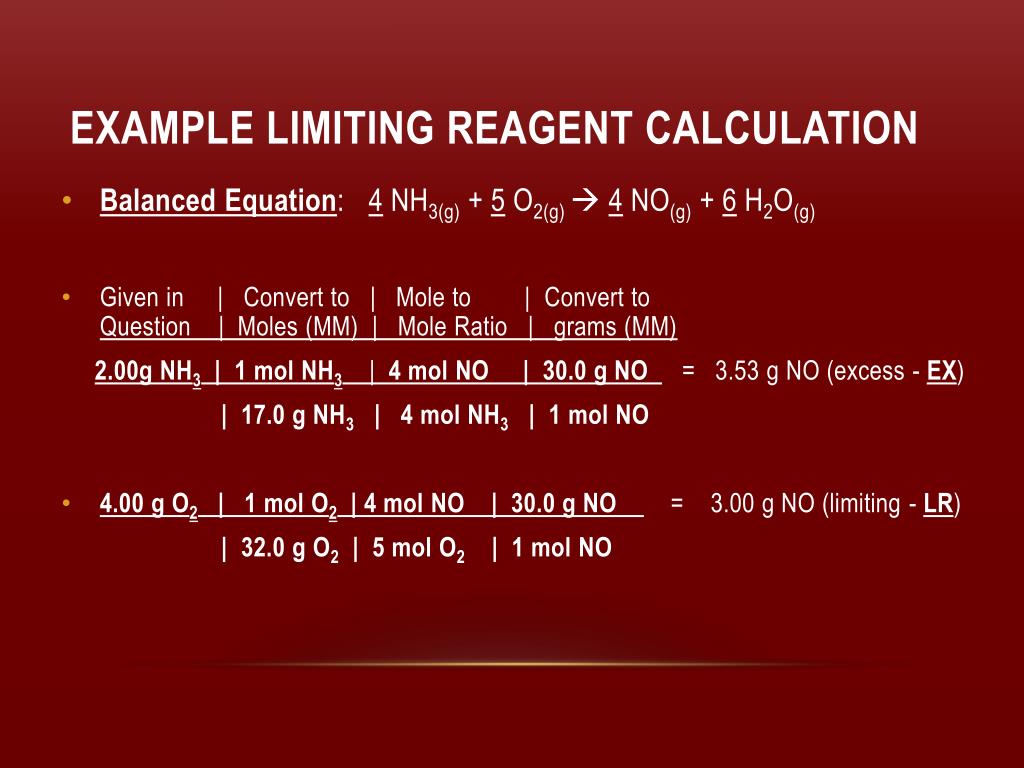
How To Calculate Limiting Reactant Slideshare
https://image1.slideserve.com/2567970/example-limiting-reagent-calculation2-l.jpg
Course Chemistry library Unit 3 Lesson 3 Limiting reagent stoichiometry Limiting reactant and reaction yields Worked example Calculating the amount of product formed from a limiting reactant Introduction to gravimetric analysis Volatilization gravimetry Gravimetric analysis and precipitation gravimetry Limiting Reagent and Percent Yield Limitin 123 and P 400 Chapter 12 Lesson 3 Key Objectives 12 3 1 EXPLAIN how the amount of product in a reaction is affected by an insufficient quantity of any of the reactants 12 3 2 EXPLAIN what the percent yield of a reaction measures Additional Resources Reading and Study Workbook Lesson 12 3
Modified from Limiting Reactant and Percent Yield Wkst pdf Blake 3 2015 2 Write the equation for the reaction of iron III phosphate with sodium sulfate to make iron III sulfate and sodium phosphate a If you perform this reaction with 25 grams of iron III phosphate and an excess of sodium sulfate how many grams of iron III 4 Al 3 O 2 2 Al 2 O 3 a Determine the theoretical yield b Determine the percent yield 4 In the reaction of Zn with HCl 140 15 g of ZnCl 2 was actually formed although the theoretical yield was 143 g What was the percent yield 5 12 5 g of copper are reacted with an excess of chlorine gas and 25 4 g of copper II chloride are obtained

Percent Yield Worksheet Answers Printable Word Searches
https://i0.wp.com/www.chemistryworksheet.com/wp-content/uploads/2022/10/percent-yield-worksheet-2.png?resize=768%2C994&ssl=1
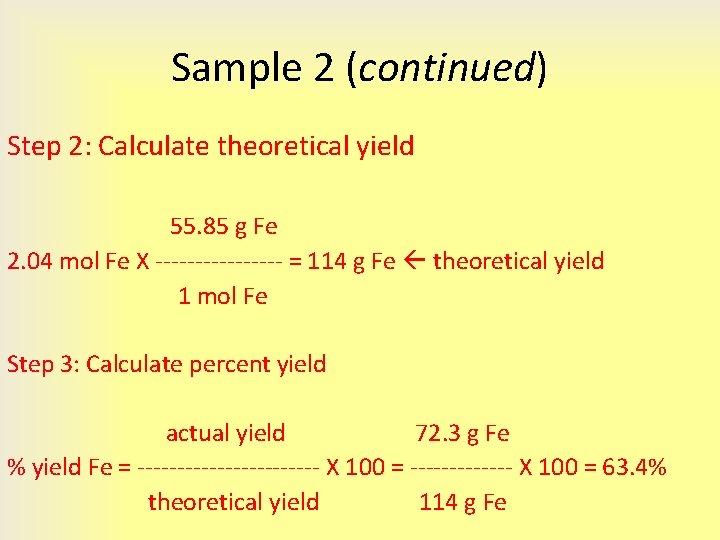
4 3 Limiting Reactant Theoretical Yield And Percent
https://slidetodoc.com/presentation_image_h/a0bc19ed740e00820be62d54ee5aae2c/image-10.jpg
12 3 Limiting Reagent And Percent Yield Worksheet Answer Key - 12 3 1 Identify the limiting reagent in a reaction 12 3 2 Calculate theoretical yield actual yield or percent yield given appropriate information Guide for Reading Build Vocabulary LINCS Have students use the LINCS strategy for the terms theoretical yield actual yield and percent yield Students should L ist the parts of a term they know I