Isotopes And Ions Worksheet Answer Key Why You have learned that not all atoms of an element are the same Variation in the number of neutrons results in different isotopes of the element In this activity we will explore another variation that can take place the loss and gain of electrons The exchange of electrons between atoms is a very common way for chemical change co take
Electrons reside in energy shells surrounding the nucleus of an atom all atoms want to completely fill their outermost energy shell 2 electrons maximum in the first shell and 8 maximum in all other shells atoms with same atomic number same protons but different of neutrons Therefore they are the same element but with different masses Isotope and ions practice worksheet name part isotopes define an isotope isotopes are atoms of the same element with different atomic masses what would use the periodic table to find in column 2 the total number of electrons that ion contains The same answer may be used more than once B 1 Al 3 A 2 D 2 Fe 3 B 10 B 3 Mg 2 C 21 H 4
Isotopes And Ions Worksheet Answer Key

Isotopes And Ions Worksheet Answer Key
https://briefencounters.ca/wp-content/uploads/2018/11/worksheet-periodic-table-answer-key-as-well-as-periodic-table-worksheet-2-answers-choice-image-periodic-table-of-of-worksheet-periodic-table-answer-key.jpg

16 Best Images Of Molecules And Atoms Worksheet Answer Key Atoms Ions
http://www.worksheeto.com/postpic/2009/09/counting-atoms-worksheet-answer-key_209039.png
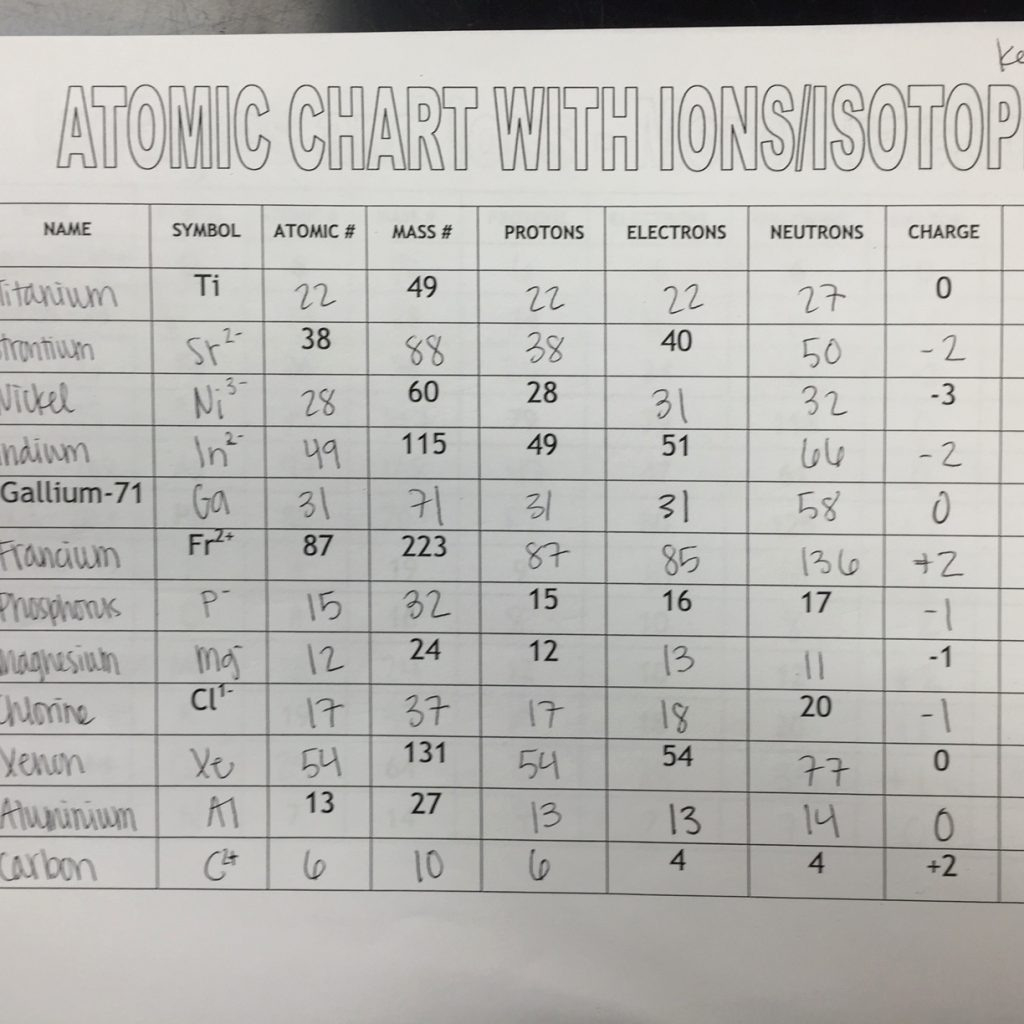
Isotope And Ions Practice Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/ions-and-isotopes-worksheet-fatmatoru.jpg
Fill in the isotope names and any missing information including isotope numbers from the chart Use your periodic table and the information provided Iodine 85 Iodine 88 of protons 53 53 of neutrons 32 35 of electrons 53 53 Iron 53 Iron 56 Description In this practice worksheet students will demonstrate understanding of isotopes and ions This should be used after the PhET simulation or similar investigation on isotope and ions Materials needed none Reformatted for 2022 as a Google Doc Older PDF version is included in the G Drive directory KEY INCLUDED in G Drive directory
Teaching Duration Reported resources will be reviewed by our team Report this resource to let us know if this resource violates TPT s content guidelines This is a worksheet designed to help students identify the number of each subatomic particle and determine if the result is an atom an ion or an isotope of the element An atom is the smallest part of an element that can exist either alone or in combination with other atoms Isotopes are atoms that have the same number of protons but different numbers of neutrons An ion is an atom or molecule with a positive or negative charge A cation is an ion with a positive charge An anion is an ion with a negative charge
More picture related to Isotopes And Ions Worksheet Answer Key
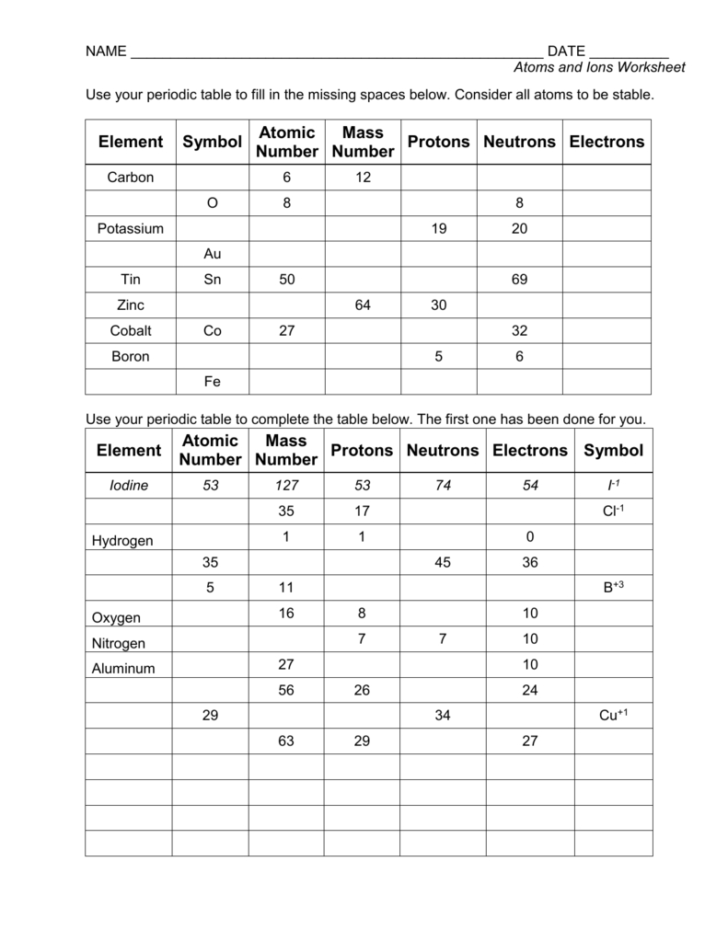
Suffix Ion Worksheet
https://db-excel.com/wp-content/uploads/2019/09/atoms-and-ions-worksheet-2-728x942.png
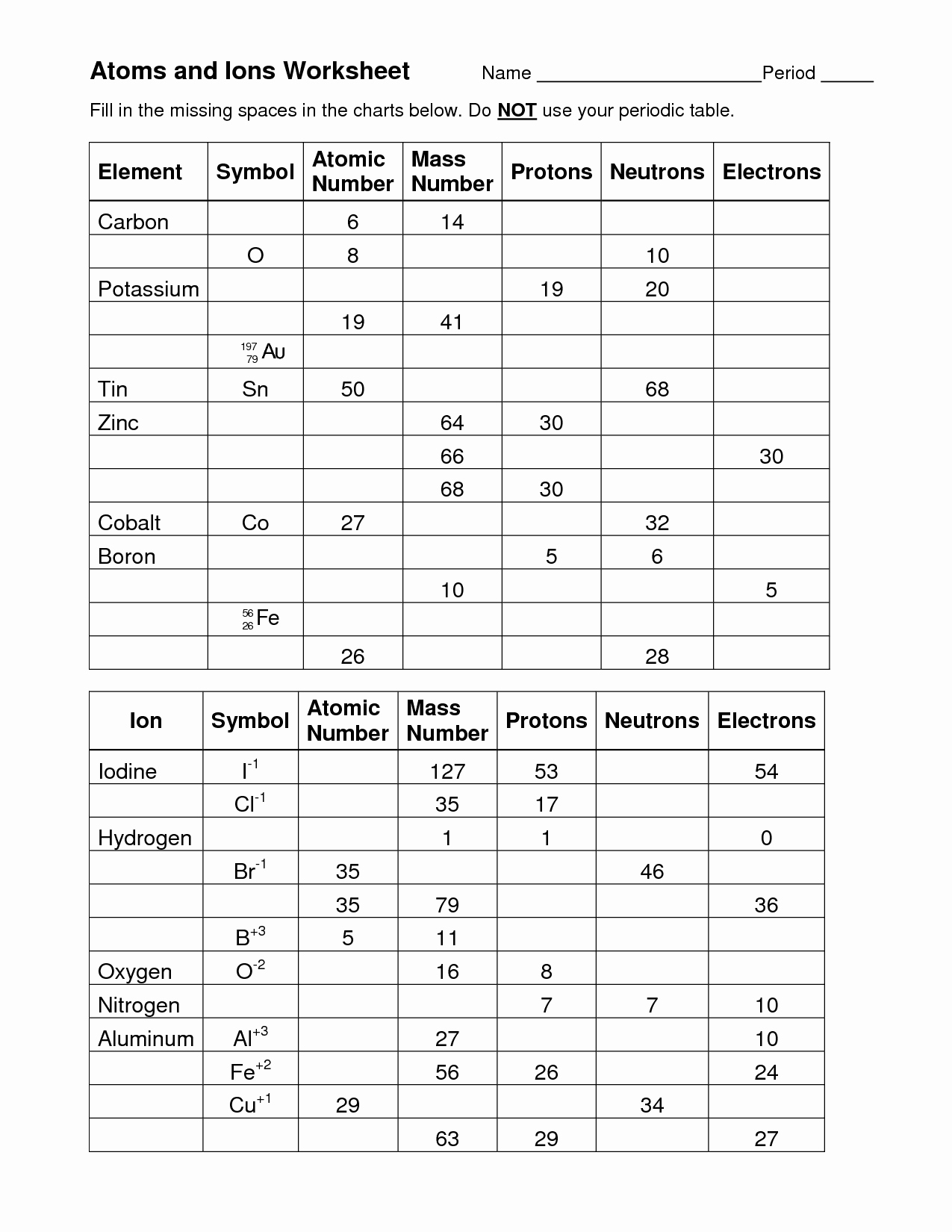
50 Isotopes Worksheet Answer Key
https://chessmuseum.org/wp-content/uploads/2019/10/isotopes-worksheet-answer-key-luxury-13-best-of-element-symbols-worksheet-answer-key-of-isotopes-worksheet-answer-key.png

Isotopes Ions And Atoms Worksheet Answers Isotope Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/isotopes-ions-and-atoms-worksheet-answers-isotope-worksheet-6.jpg
Atoms The number of protons in an atom determines the identity of the atom Atomic Protons In a neutral atom the number of positive protons equals the number of negative electrons Protons Electrons Protons and neutrons both have a mass of 1 amu The mass of the electron is negligible compared to the mass of the proton and neutron This is a worksheet of extra practice problems for students who struggled with the ions and ion notation worksheet and or the isotopes and isotope notation worksheet Students are given a simple table that gives limited information about an isotope or ion and they fill in the rest Essential Concepts Ions ion notation electrons anions
Astatine 211 8 Fill in the following table 9 Calculate the average atomic mass of chlorine if its isotopes and abundances are as follows Show all work 1 Describe the general arrangement of subatomic particles in the atom Electrons surround the nucleus protons and neutrons are in the nucleus 2 Atoms are made up of protons and neutrons located within the nucleus with electrons in orbitals surrounding the nucleus Protons and neutrons have approximately the same mass about 1 67 10 24 grams Scientists arbitrarily define this amount of mass as one atomic mass unit amu or one Dalton as Table 2 2 shows
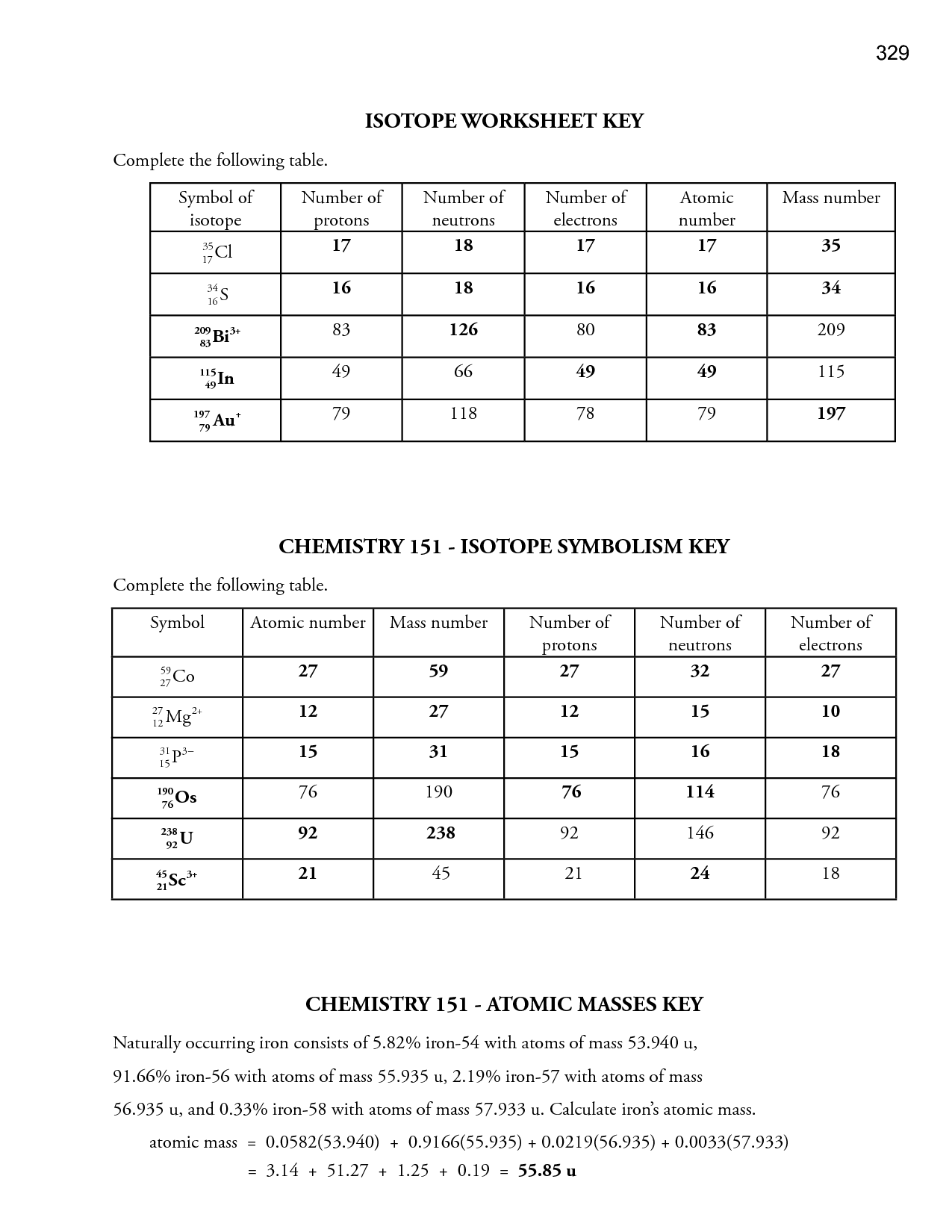
13 Best Images Of Atomic Structure Practice Worksheet Periodic Table
http://www.worksheeto.com/postpic/2009/12/isotopes-worksheet-answer-key_212387.png
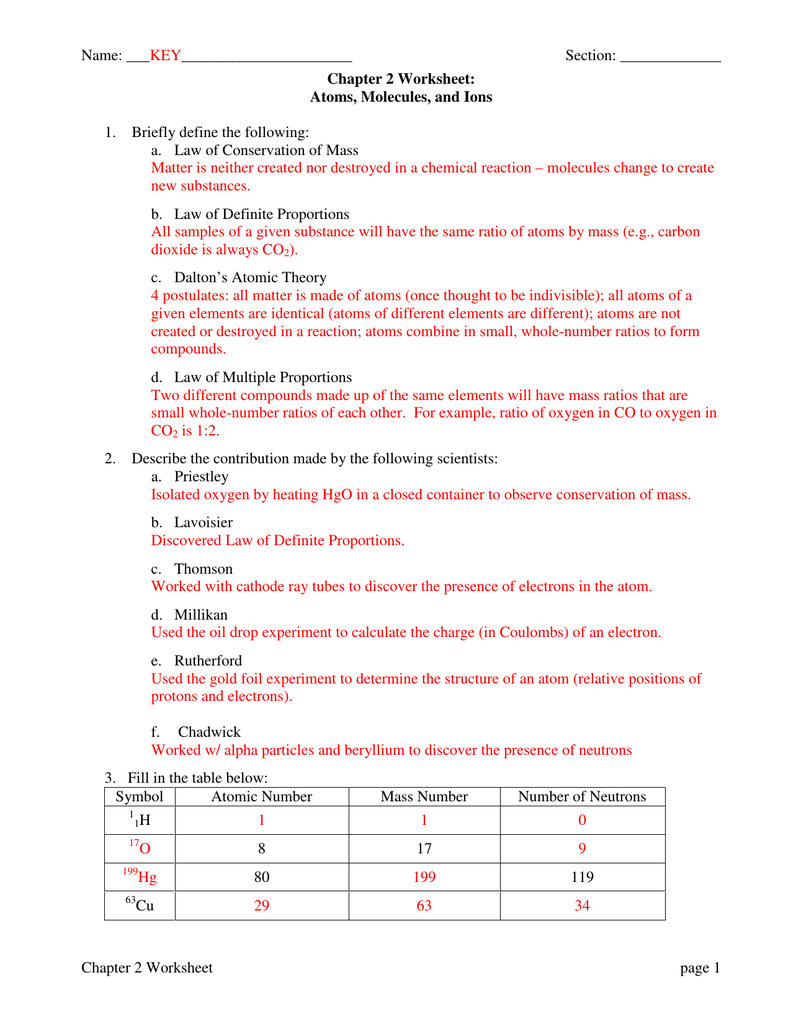
Atoms And Ions Worksheet Ivuyteq
https://s1.studyres.com/store/data/001961937_1-ce322185ddc89110a1e2a883fa1fe3f6.png
Isotopes And Ions Worksheet Answer Key - Description In this practice worksheet students will demonstrate understanding of isotopes and ions This should be used after the PhET simulation or similar investigation on isotope and ions Materials needed none Reformatted for 2022 as a Google Doc Older PDF version is included in the G Drive directory KEY INCLUDED in G Drive directory