Isotopes And Average Atomic Mass Worksheet 2 5 Isotopes and Average Atomic Mass is shared under a not declared license and was authored remixed and or curated by LibreTexts Isotopes of an element are atoms with the same atomic number but different mass numbers isotopes of an element therefore differ from each other only in the number of neutrons within the nucleus
Average Atomic Mass Worksheet show all work 1 Rubidium is a soft silvery white metal that has two common isotopes 85Rb and 87Rb If the abundance of 85Rb is 72 2 and the abundance of 87Rb is 27 8 what is the average atomic mass of rubidium 2 Uranium is used in nuclear reactors and is a rare element on earth 7 Boron exists in two isotopes boron 10 and boron 11 Based on the atomic mass which isotope should be more abundant Answer The atomic mass of boron is 10 811 therefore boron 11 is more abundant because the mass number is closer to the atomic mass 8 Lithium 6 is 4 abundant and lithium 7 is 96 abundant What is the average mass of
Isotopes And Average Atomic Mass Worksheet

Isotopes And Average Atomic Mass Worksheet
http://www.housview.com/wp-content/uploads/2018/02/worksheet_templates___average_atomic_mass_isotope_worksheet_5.jpg

Isotopes And Average Atomic Mass Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/handout-03-average-atomic-mass-1.png
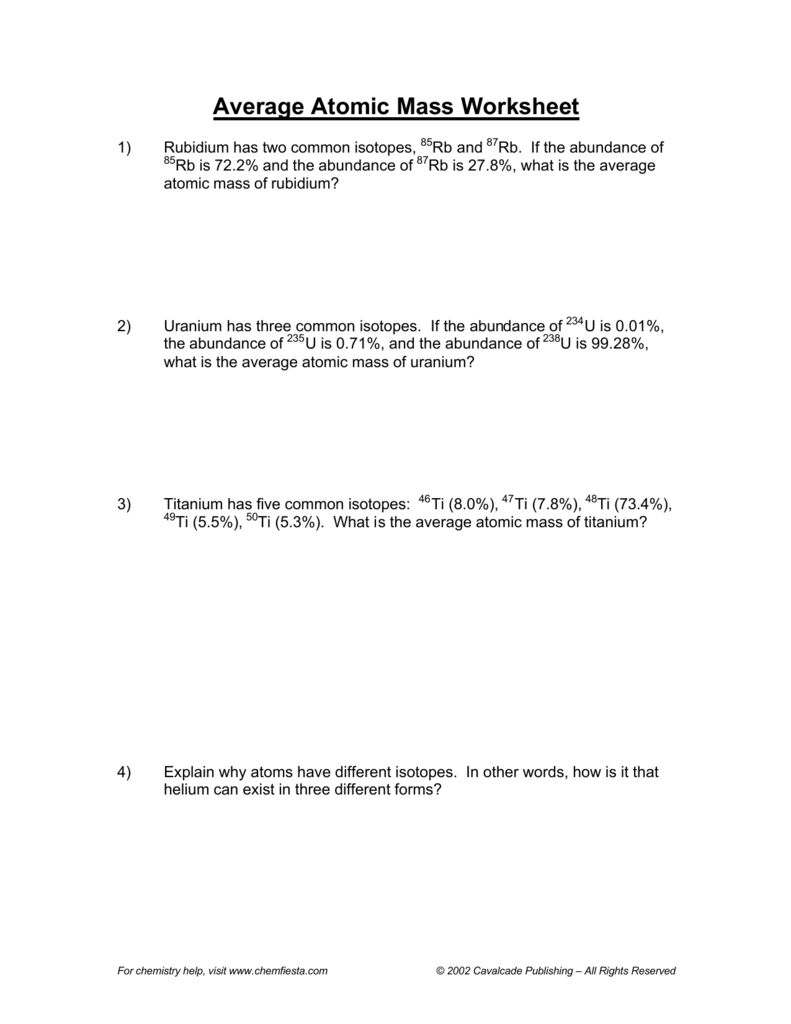
Average Atomic Mass Worksheet
https://s3.studylib.net/store/data/008342061_1-f57ef67d289a7496bec610a04719b82e.png
Astatine 211 8 Fill in the following table 9 Calculate the average atomic mass of chlorine if its isotopes and abundances are as follows Show all work 1 Describe the general arrangement of subatomic particles in the atom Electrons surround the nucleus protons and neutrons are in the nucleus 2 PROBLEM 2 3 4 2 3 4 Average atomic masses listed by IUPAC are based on a study of experimental results Bromine has two isotopes 79 Br and 81 Br whose masses 78 9183 and 80 9163 amu and abundances 50 69 and 49 31 were determined in earlier experiments Calculate the average atomic mass of Br based on these experiments
To calculate a weighted average of its isotope masses a Use the equation in question 1 to calculate the atomic mass of an element that has two isotopes each with 50 00 abundance One isotope has a mass of 63 00 amu and the other has a mass of 68 00 amu b Recalculate the atomic mass if instead there is 80 00 of the 63 00 amu isotope and Because the sum of the numbers of protons and neutrons equals the mass number 127 the number of neutrons is 74 127 53 74 Since the iodine is added as a 1 anion the number of electrons is 54 53 1 54 Exercise 2 2 1 2 2 1 An ion of platinum has a mass number of 195 and contains 74 electrons
More picture related to Isotopes And Average Atomic Mass Worksheet

02 06 Average Atomic Mass Worksheet 2
https://s3.studylib.net/store/data/008549995_1-ed2f631999dd462990e0c96ed9c082ca.png
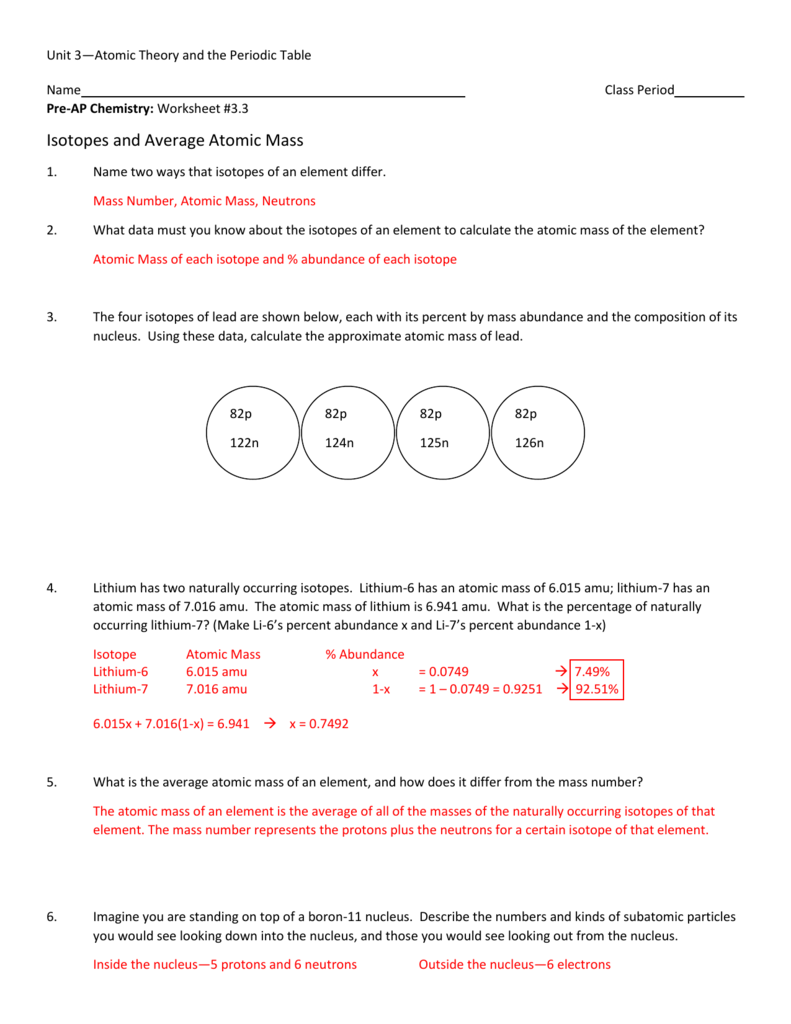
Isotopes And Average Atomic Mass
https://s3.studylib.net/store/data/008720700_1-5ebe2b05fecfe6e076a34d89445b2234.png

Average Atomic Mass
https://s3.studylib.net/store/data/008720699_1-0b71c6cf42948720280d2eb69c81864d.png
Chemistry Average Atomic Mass Worksheet Calculate the average atomic mass for each element based on the natural abundance of its isotopes 1 Find the average atomic mass for Li if 7 5 of Li atoms are 6Li with a mass of 6 0151223 amu and 92 5 are 7Li with a mass of 7 0160041 amu 2 Find the average atomic mass for B if 19 9 of B atoms are Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element Are all atoms of an element the same How can you tell one isotope from another Use the sim to learn about isotopes and how abundance relates to the average atomic mass of an element
Average Atomic Mass Worksheet 1 Rubidium has two common isotopes 85Rb and 87Rb If the abundance of 85Rb is 72 2 and the abundance of 87Rb is 27 8 what is the average atomic mass of rubidium 2 Uranium has three common isotopes If the abundance of 234U is 0 01 the abundance of 235U is 0 71 and the abundance of 238U is 99 28 Isotopes and average atomic mass as concepts allow for the specific discussion of elements and their atoms and this quiz worksheet combo will help you test your understanding of these concepts
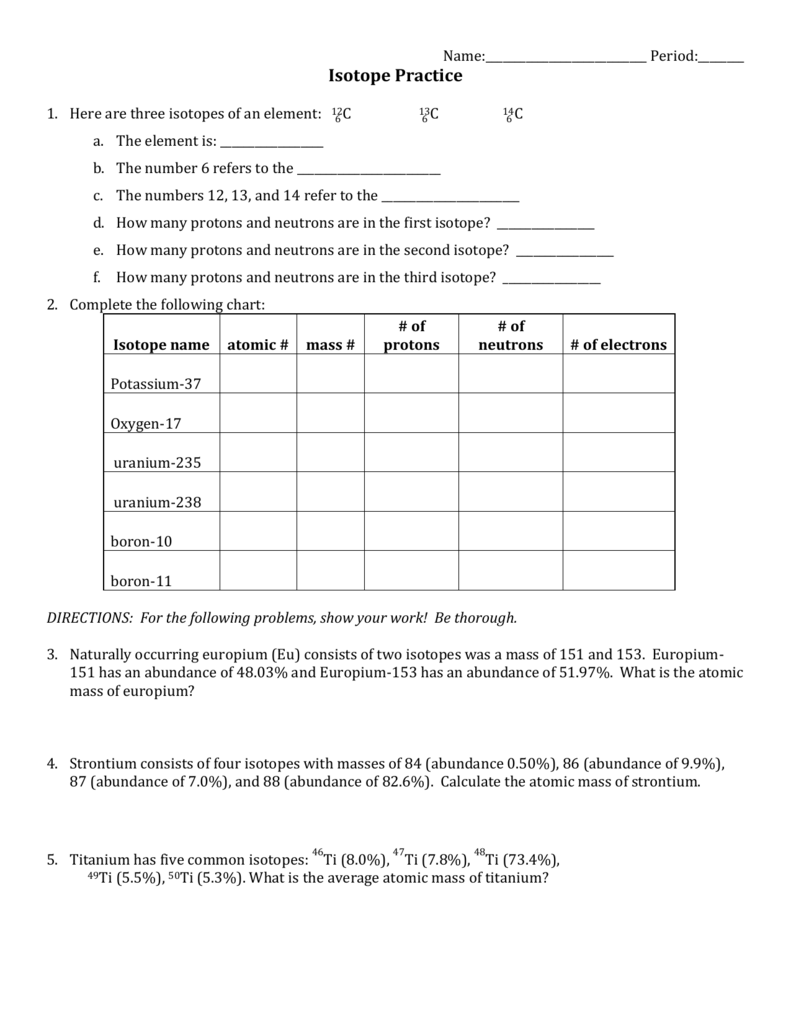
Worksheet Atomic Mass Worksheet Grass Fedjp Worksheet Study Site
http://s3.studylib.net/store/data/008034703_1-985fdc7d7e397ddeb30ff240e12fda1a.png

Average Atomic Mass Worksheet Gail Centeno Library Formative
https://dm2ec218nt2z5.cloudfront.net/2020-10-15/5f3c350fe96da246d6782d3a/5f885eaea968be111c97aa8c_AverageAtomicMassWorksheet.pdf/page-2.png
Isotopes And Average Atomic Mass Worksheet - Find the average atomic mass for Mg if 78 99 of Mg atoms are 24Mg with a mass of 23 9850419 amu 10 00 are 25Mg with a mass of 24 9858370 amu and 11 01 are 26Mg with a mass of 25 9825930 amu Calculate the average atomic mass for each element based on the natural abundance of its isotopes Learn with flashcards games and more for free