Heat Of Fusion And Heat Of Vaporization Worksheet Molar Heat of Fusion and Molar Heat of Vaporization Worksheet 100 0 176 C Calculate the heat needed to change 75 0 grams of ice at 40 0 176 C to water at 20 0 176 C The amount of heat required is equal to the sum of these three steps
Believe it or not but you encounter the heat of fusion and heat of vaporization every day Find out what you know by completing the worksheet and quiz pairing 1 What is the molar heat of solidification for water 2 How much energy is released to the environment by 50 0 grams of condensing water vapor 3 Is melting endothermic or exothermic Explain 4 Calculate the amount of heat needed to melt 35 0 g of ice at 0 186 C Express your answer in kilojoules 5
Heat Of Fusion And Heat Of Vaporization Worksheet

Heat Of Fusion And Heat Of Vaporization Worksheet
https://study.com/academy/practice/quiz-worksheet-heat-of-fusion-heat-of-vaporization.jpg
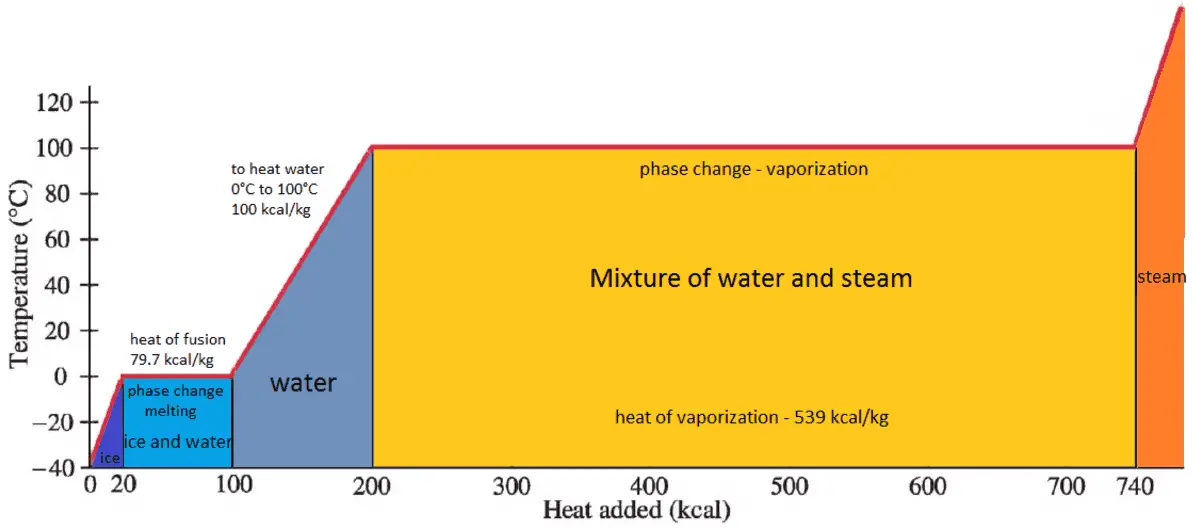
What Is Latent Heat Of Vaporization Definition
https://thermal-engineering.org/wp-content/uploads/2019/05/Phease-Changes-Heat-of-Vaporization-Water-min.png
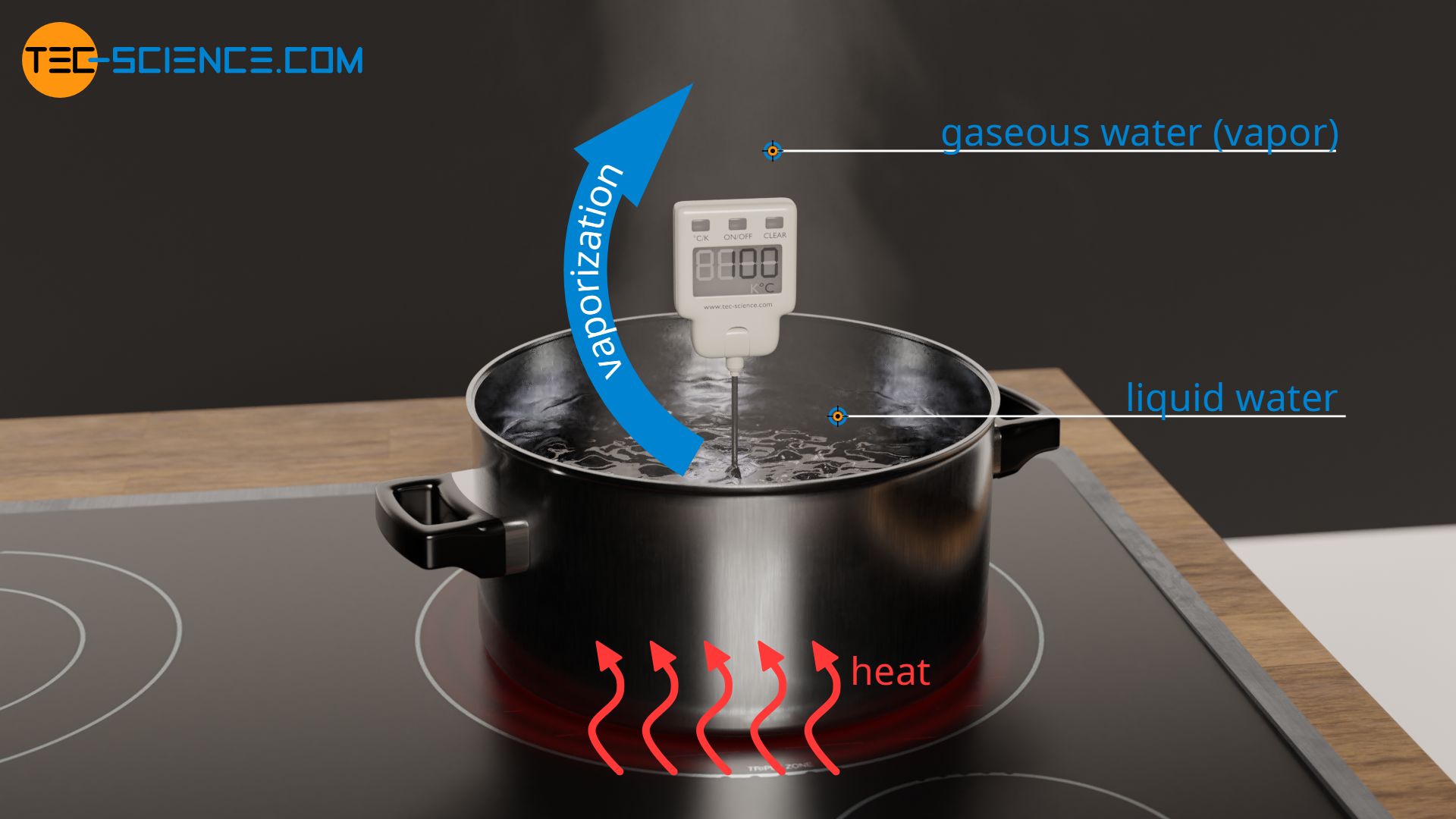
Specific Latent Heat Of Vaporization Tec science
https://www.tec-science.com/wp-content/uploads/2021/05/en-thermodynamics-specific-latent-heat-vaporization-enthalpy-determination-experiment-water.jpg
How much heat is required to heat 1 5 kg of water from 50 186 C to 100 186 C and then to turn it into steam at 100 186 C This problem requires formulas 3 and 2 above 1 What is the molar heat of solidification for water 2 How much energy is released to the environment by 50 0 grams of condensing water vapor 3 Is melting endothermic or exothermic Explain 4 Calculate the amount of heat needed to melt 35 0 g of ice at 0 amp ordm C Express your answer in kilojoules 5
1 What is latent heat 2 Why does the temperature of H2O not increase when it is boiling Explain your answer by drawing a heating cooling curve for water 3 Describe the difference between latent heat of fusion versus latent heat of vaporization Which process involves energy being absorbed 4 Heat with Phase Change Worksheet 1 How many joules are required to heat 250 grams of liquid water from 00 to 1000 C 2 How many joules are required to melt 100 grams of water 3 How many joules are required to boil 150 grams of water
More picture related to Heat Of Fusion And Heat Of Vaporization Worksheet

Four Differencebetween Latent Heat Of Fusion And Latent Heat Of
https://hi-static.z-dn.net/files/daf/c84a2ede2ffeaf20ff7992bc21bdcfee.jpg

Latent Heat Of Fusion And Vaporisation YouTube
https://i.ytimg.com/vi/ZJl00IttILo/maxresdefault.jpg

Mr Murray s Website Two Dim Motion
http://www.cstephenmurray.com/Acrobatfiles/aphysics/NotesAndExamples/HeatAndThermo/UnderstandingHeat.gif
The Heat of Fusion and Vaporization Calculations Worksheet consists of two pages Page 1 Define Fusion Write the Formula to Calculate Heat of Fusion Write the Heat of Fusion for water Apr 17 2021 nbsp 0183 32 In the following practice problems by applying latent heat of vaporization some heat problems involving phase changes are answered The heat of Vaporization Problems Problem 1 A 10 g chunk of liquid lead at 1750 176 C takes 8580 joules of heat to turn into 10 g of gaseous lead at 1750 176 C What is the latent heat of vaporization of the lead
The purpose of this experiment is to measure the heats of fusion and vaporization of water To determine Lf experimentally we will add a measured mass of ice at the freezing point to a measured mass of liquid water in a calorimeter of known mass and known specific heat capacity both of which are at a known initial temperature 1 Heat the water from 30 C to 100 C Q sm T 2 Vaporize the water Q m Hv The total heat needed will be the sum of these two calculations Q sm T m Hv 2 Calculate the heat needed to change 75 0 grams of ice at 40 0 C to water at 20 0 C The specific heat of solid ice is 2 04 J g C and the heat of fusion of ice is 333 6 J g
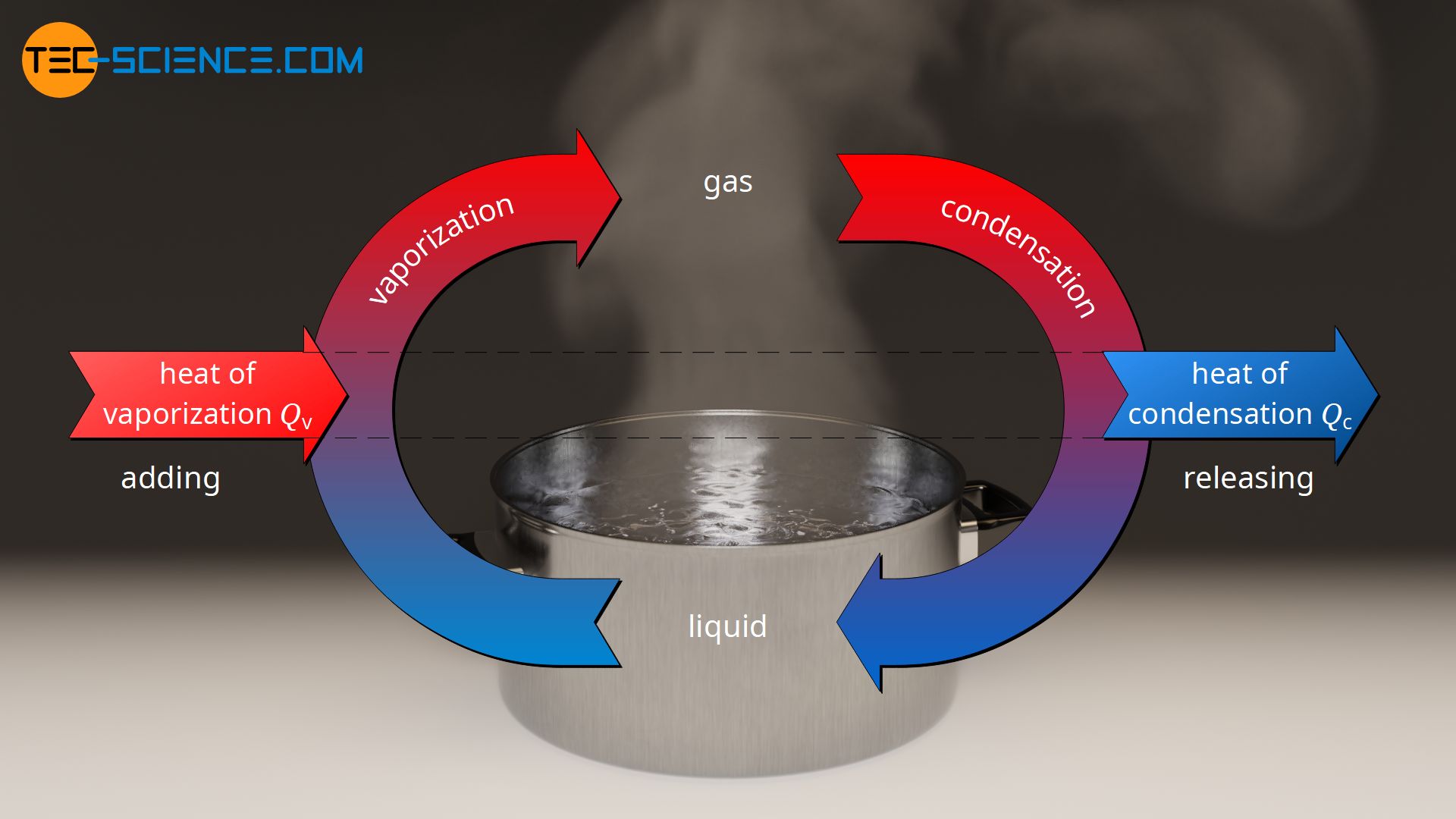
Specific Latent Heat Of Condensation Tec science
https://www.tec-science.com/wp-content/uploads/2021/05/en-thermodynamics-specific-latent-heat-condensation-enthalpy-vaporization-energy-flow.jpg

Entropy Of Vaporization And Fusion YouTube
https://i.ytimg.com/vi/oyp9qmy5vOs/maxresdefault.jpg
Heat Of Fusion And Heat Of Vaporization Worksheet - Showing 8 worksheets for Heat Of Fusion And Vaporization Worksheets are 270 2 1 heats of fusion and vaporization theory Molar heat of fusion and mol