Heat Of Fusion And Vaporization Worksheet Pdf What is the heat of fusion of zinc How much energy is needed to heat a 125 g sample of water from 20 176 C to 100 176 C Note that this is a specific heat problem How much energy does it take to heat 125 g of steam from 100 176 C to 110 176 C Specific heat of steam 2 01 J g 176 C
Dec 11 2014 nbsp 0183 32 Enthalpy Heat of Fusion H fus amp of Vaporization H vap Worksheet These Enthalpies Heats are the amount of energy required or given off BY ONE GRAM of the substance when it is changing from one phase to another During this phase change the temperature remains constant so there s no temp change in the equation Heat of Vaporization and Heat of Fusion Worksheet 1 How much heat is required to melt 360 g of solid water Important constant Hfus of water is 334 J g 2 How much heat is required to vaporized 24 g of liquid water Hvap of water is 2257 J g 3 For a 500g block of lead Pb to melt how much energy is needed Hfus of lead is 23 J g 4
Heat Of Fusion And Vaporization Worksheet Pdf

Heat Of Fusion And Vaporization Worksheet Pdf
https://i2.wp.com/image2.slideserve.com/5070642/heat-of-fusion-and-vaporization-from-heating-graphs1-l.jpg

Heat Of Fusion And Vaporization Worksheet Printable Word Searches
https://i2.wp.com/s3.studylib.net/store/data/009195845_1-11e7dd132096bde7009590aab5bc9d0d-768x994.png

Heat Of Fusion Heat Of Vaporization Notes Made By Teachers
https://media.madebyteachers.com/wp-content/uploads/2023/01/15144311/cover-heat-of-fusion-1013x1024.png
1 How much heat is required to melt 4 kg of ice at 0 186 C Remember there is no T in a phase change 2 How much heat is required to turn 12 kg of water at 100 186 C into steam at the same temperature Again there is no T in a phase change 3 How much heat is required to heat 1 5 kg of water from 50 186 C to 100 186 C and then to turn it into Showing 8 worksheets for Heat Of Fusion And Vaporization Worksheets are 270 2 1 heats of fusion and vaporization theory Molar heat of fusion and mol
What is the molar heat of solidification for water 2 How much energy is released to the environment by 50 0 grams of condensing water vapor 3 Is melting endothermic or exothermic Explain 4 Calculate the amount of heat needed to melt 35 0 g of ice at 0 amp ordm C Express your answer in kilojoules 5 The purpose of this experiment is to measure the heats of fusion and vaporization of water To determine Lf experimentally we will add a measured mass of ice at the freezing point to a measured mass of liquid water in a calorimeter of known mass and known specific heat capacity both of which are at a known initial temperature
More picture related to Heat Of Fusion And Vaporization Worksheet Pdf
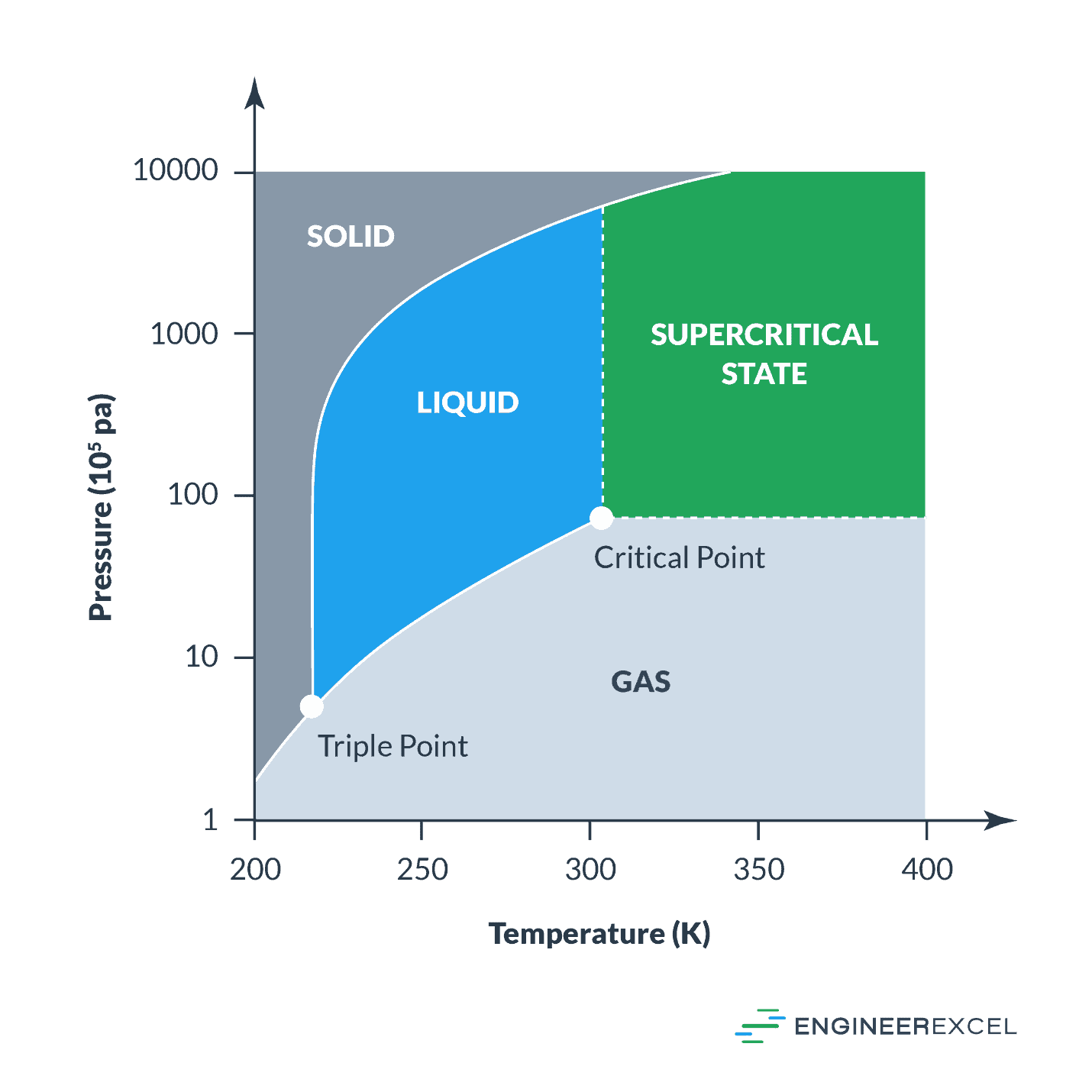
Heat Of Vaporization Explained EngineerExcel
https://engineerexcel.com/wp-content/uploads/2023/03/heat-of-vaporization.png
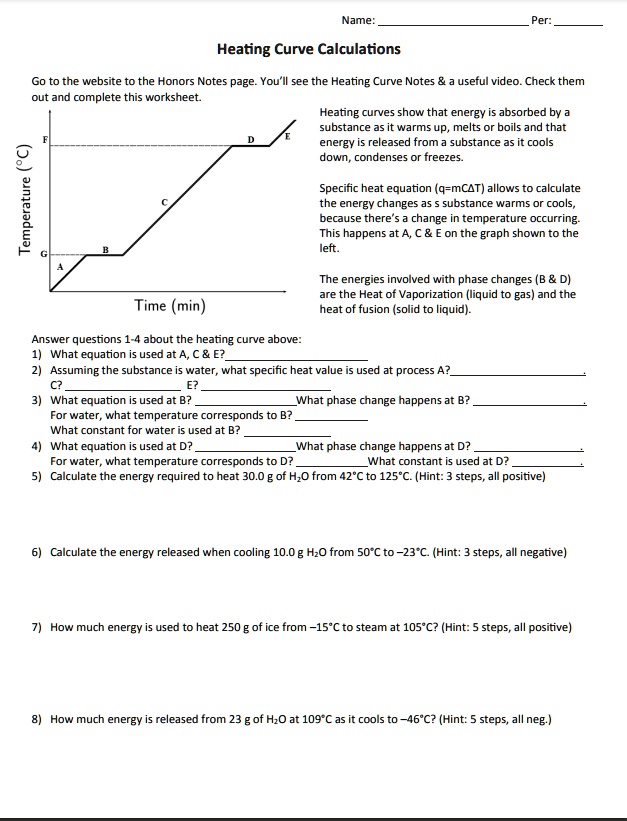
Heat Of Fusion And Vaporization Worksheet Key
https://cdn.numerade.com/ask_images/c155d905a3e24ebb95f7bfa04c26c639.jpg
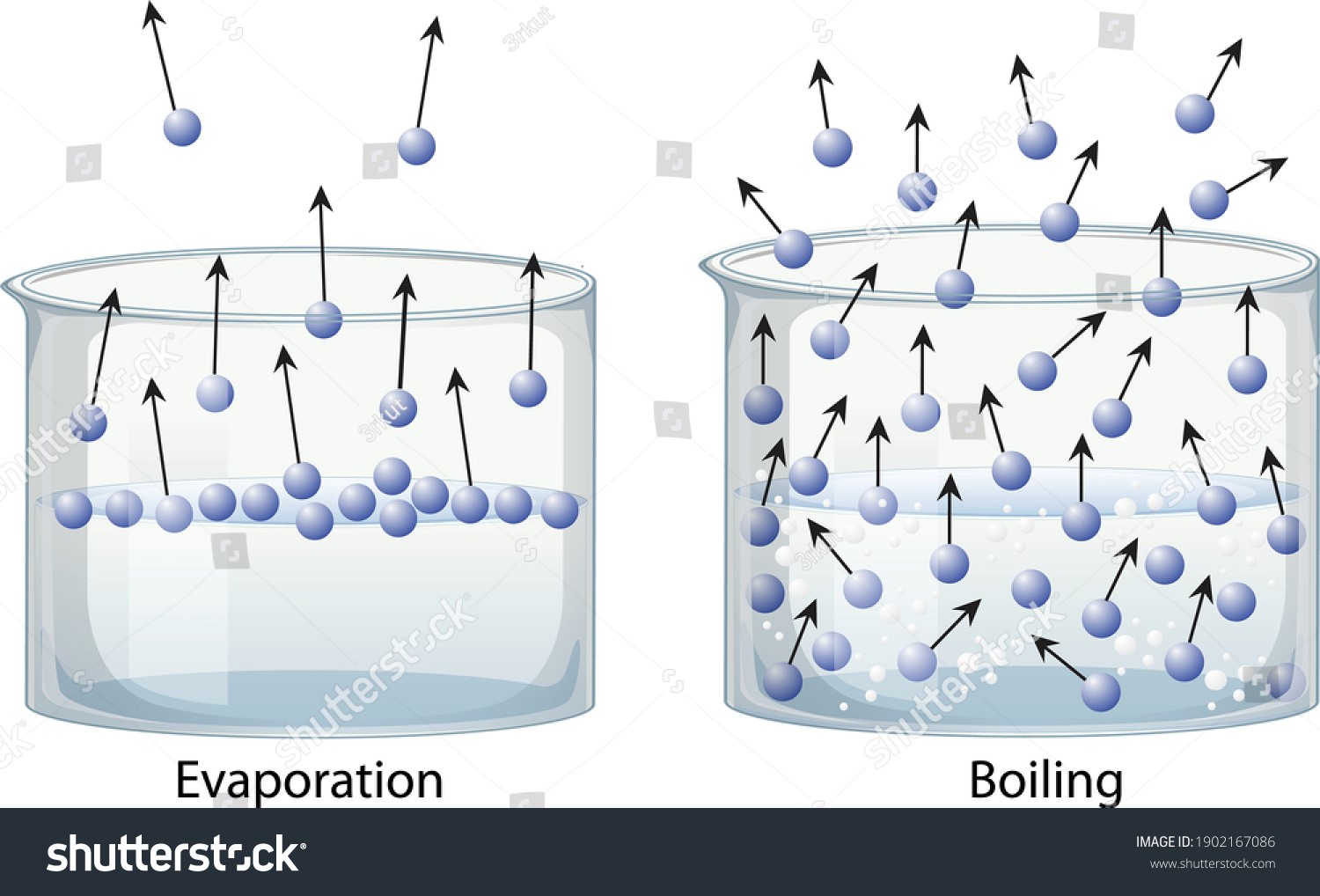
Vaporization Types Examples And Factors Affecting The Rate Of
https://library.88guru.com/wp-content/uploads/2023/02/Evaporation-vs-Boiling.jpg
In this worksheet you will be using the following equation Thermal energy for a change in state J mass kg specific latent heat J kg Useful values Molar Heat of Fusion and Molar Heat of Vaporization Worksheet 1 Calculate the heat needed to change 3 00 x 10 2 grams of water at 25 0 176 C to steam at 100 0 176 C 2 Calculate the heat needed to change 75 0 grams of ice at 40 0 176 C to water at 20 0 176 C The amount of heat required is equal to the sum of these three steps 3
[desc-10] [desc-11]

Latent Heat Of Fusion And Vaporisation YouTube
https://i.ytimg.com/vi/ZJl00IttILo/maxresdefault.jpg
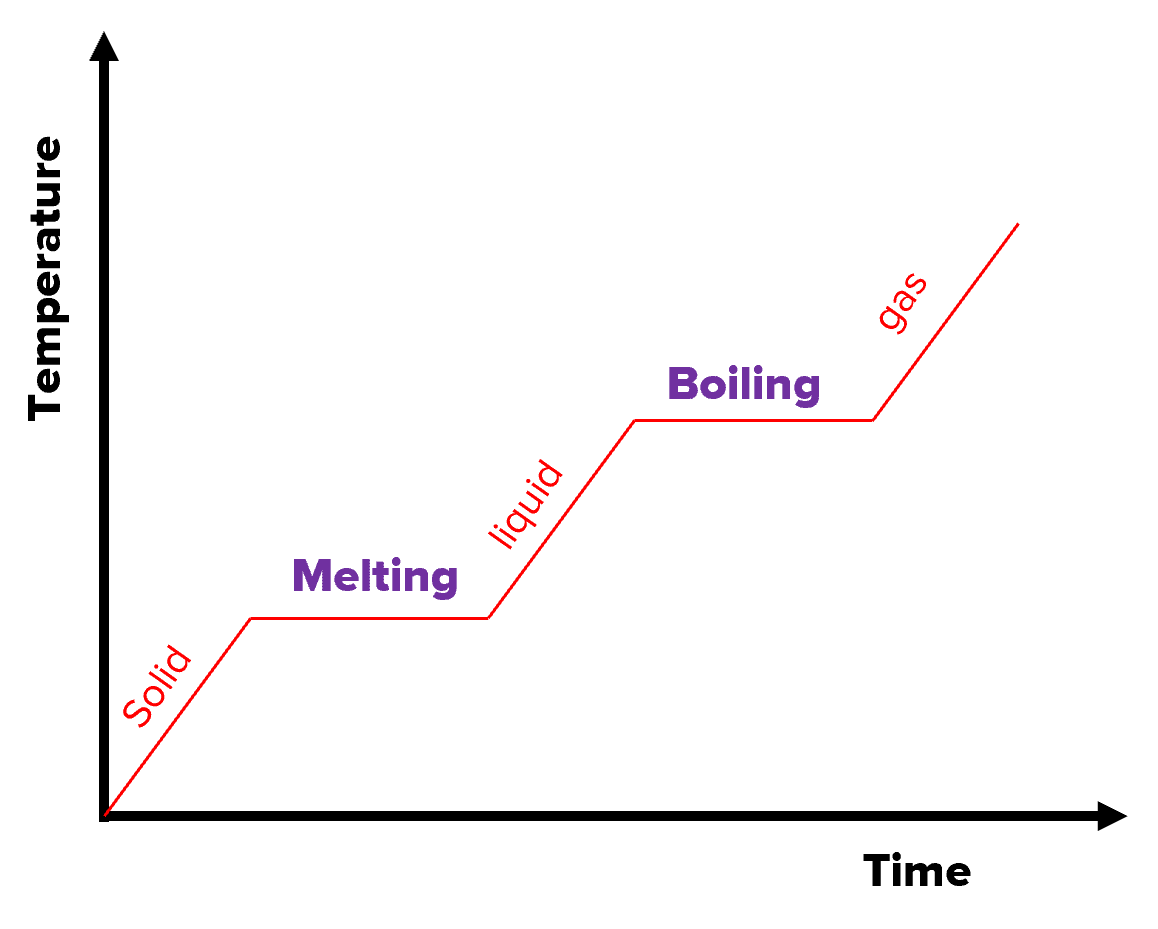
Specific Latent Heat Questions And Revision MME
https://mmerevise.co.uk/wp-content/uploads/2022/10/Heating-Graph.png
Heat Of Fusion And Vaporization Worksheet Pdf - The purpose of this experiment is to measure the heats of fusion and vaporization of water To determine Lf experimentally we will add a measured mass of ice at the freezing point to a measured mass of liquid water in a calorimeter of known mass and known specific heat capacity both of which are at a known initial temperature