Limiting Reagent Worksheet 2 Answers Practice Problems Limiting Excess Reagents 1 Forthe reaction 2S s 302 g 2S03 g if6 3 g ofS is reacted with 10 0 g of02 show by calculation which one will be the limiting reactant 2 Forthe reaction CaC03 s 2HCl aq CaC12 aq CO2 g H20 l 68 1 g solid CaC03 is mixed with 51 6 g HCl What number ofgrams ofCO2 will be
Limiting Reagent Worksheet 2 1 Consider the reaction a 80 0 grams of iodine V oxide I2O5 reacts with 28 0 grams of carbon monoxide CO CO is limiting Determine the mass of iodine I2 which could be produced 50 7 g b If in the above situation only 0 160 moles of iodine I2 was produced ii what percentage yield of iodine was Q1 Given the following reaction hint balance the equation first C a O H 2 H 2 S O 4 C a S O 4 2 H 2 O If you start with 14 82 g of C a O H 2 and 16 35 g of H 2 S O 4 a determine the limiting reagent b determine the number of moles of H 2 O produced c determine the number of grams of C a S O 4 produced
Limiting Reagent Worksheet 2 Answers

Limiting Reagent Worksheet 2 Answers
https://db-excel.com/wp-content/uploads/2019/09/limiting-reagent-worksheet-1-14.jpg

Limiting Reagent Worksheet 1 Answers In 2022 Persuasive Writing
https://i.pinimg.com/originals/52/dd/4d/52dd4d3cd34dbca71302417c2846ac71.png

Limiting Reagent
https://s3.studylib.net/store/data/025270016_1-507111ac20294a235df264dbbe41c428-768x994.png
If you start with 14 82 g of Ca OH 2 C a O H 2 and 16 35 g of H2SO4 H 2 S O 4 a determine the limiting reagent b determine the number of moles of H2O H 2 O produced c determine the number of grams of CaSO4 C a S O 4 produced d determine the number of grams of excess reagent left 1 make sure the equation is balanced Course Chemistry library Unit 3 Lesson 3 Limiting reagent stoichiometry Limiting reactant and reaction yields Worked example Calculating the amount of product formed from a limiting reactant Introduction to gravimetric analysis Volatilization gravimetry Gravimetric analysis and precipitation gravimetry
2 and Ca OH 2 a Write a balanced equation for this reaction See answer b What mass of pure CaC 22 must be added to excess water to produce 41 6 g C H 2 41 6 g C 22 H is 41 6 26 0 1 60 mol 1 1 stoich comes from 1 60 mol CaC 2 mass of CaC 22 1 60 64 0 102 g pure CaC c Calcium carbide is commonly less than 100 pure Practice Problems Limiting Reagents Answer Key Take the reaction NH 3 O 2 NO H 2 O In an experiment 3 25 g of NH 3 are allowed to react with 3 50 g of O 2 a Which reactant is the limiting reagent O 2 b How many grams of NO are formed 2 63 g NO c How much of the excess reactant remains after the reaction 1 76 g NH 3 left
More picture related to Limiting Reagent Worksheet 2 Answers
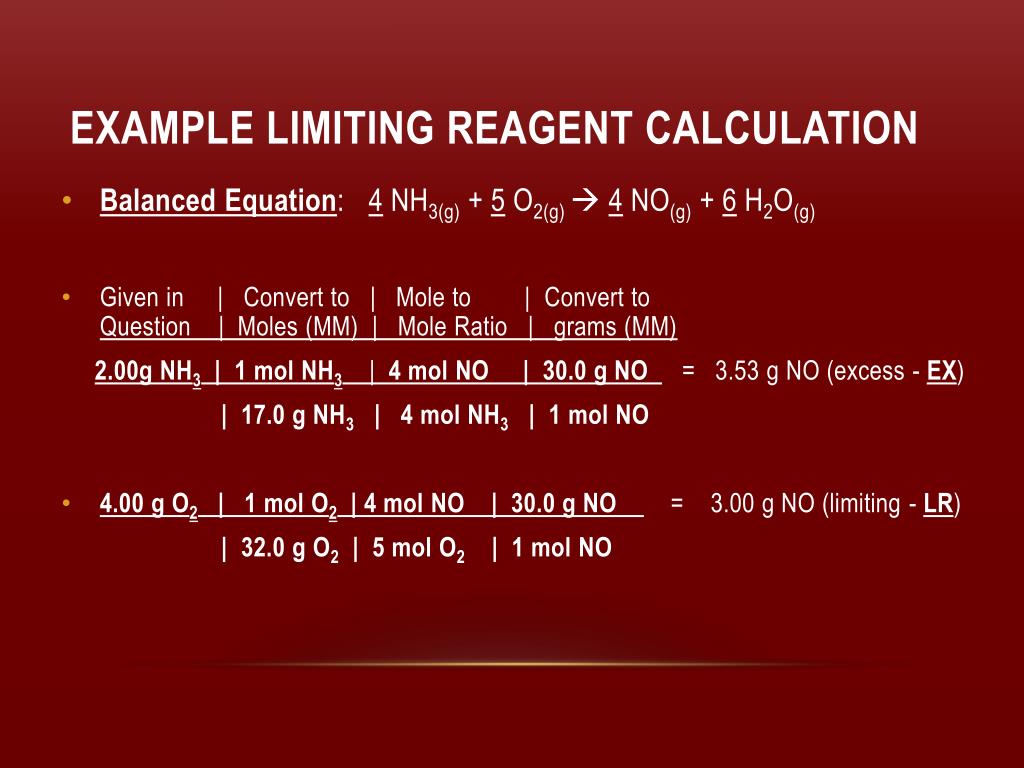
PPT Limiting Reagent PowerPoint Presentation Free Download ID 2567970
https://image1.slideserve.com/2567970/example-limiting-reagent-calculation2-l.jpg

Limited Reagent Worksheet Answers Printable Word Searches
https://i2.wp.com/i.ytimg.com/vi/0PUCi58THkU/maxresdefault.jpg

Limiting Reagent Worksheet Answers Worksheets Free Worksheets Samples
https://www.housview.com/wp-content/uploads/2018/02/limiting_reagent_worksheet_answers_worksheets_8.gif
And Physics Worksheet 2 Limiting Reagents 1 Given the following reaction hint balance the equation first Na2SO4 AgNO3 g NaNO3 Ag2SO4 If you start with 60 mL of a 3 0 M Na2SO4 solution and 40 mL of a 3 0 M AgNO3 solution a determine the limiting reagent b using the information from part a determine the theoretical yield of Ag2SO4 Limiting Reagent Worksheet W 324 1 Write the balanced equation for the reaction that occurs when iron II chloride is mixed with sodium phosphate forming iron II phosphate and sodium chloride 2 If 23 grams of iron II chloride reacts with 41 grams of sodium phosphate what is the limiting reagent How much sodium chloride can be formed
Calculate the mass in g of acetylene that can be prepared by the reaction of 6 4 g CaC 2 with 10 5 g H 2 O 2 PRACTICE PROBLEM The image below shows the reaction between A green spheres and B 2 orange spheres to form AB 3 i Identify the limiting reagent and ii calculate the moles of AB 3 formed using 1 0 mol of A and 1 0 mol of B 2 03 Equilibrium Constant Worksheet 2 Answers Spring 2019 Exam 2 Preview text Limiting Reagent Worksheet 1 1 Given the following reaction Balance the equation first Limiting Reagent Worksheet 1 1 Given the following reaction Balance the equation first C 3 H 8 5 O 2 3 CO 2 4H 2 O a If you start with 14 g of C 3 H 8

Maxresdefault jpg
http://i1.ytimg.com/vi/qXWRNqkpB4c/maxresdefault.jpg

Worksheet For Limiting Reagent Docsity
https://static.docsity.com/documents_first_pages/2021/04/20/edf30e702b0257f8e7885ecccfae137e.png?v=1630065724
Limiting Reagent Worksheet 2 Answers - A determine the limiting reagent b using the information from part a determine the theoretical yield of Ag2SO4 A g 2 S O 4 c determine the number of grams of excess reagent left 2 Given the following reaction Ca OH 2 HClO4 H2O Ca ClO4 2 C a O H 2 H C l O 4 H 2 O C a C l O 4 2