Stoichiometry Limiting Reagent Worksheet 2 Answers 1 Consider the following reaction NH4NO3 Na3PO4 NH4 3PO4 NaNO3 Which reactant is limiting assuming we started with 30 0 grams of ammonium nitrate and 50 0 grams of sodium phosphate What is the mass of each product that can be formed What mass of the excess reactant s is left over 2 Consider the following reaction
To determine the amounts of product either grams or moles you must start with the limiting reagent Use the amount that you have not the amount you need To determine the grams of excess reagent subtract the amount you need from the amount that you have then using the molar mass convert the moles left to grams Detailed Solutions to Limiting Reagent Problems 1 Disulfur dichloride is prepared by direct reaction of the elements S 82 s 4 Cl g 4 S 2 Cl 2 l What is the maximum amount of S 22 Cl that could be made by the reaction of 64 0 g of sulfur with 142 g of chlorine What quantity of which reagent would remain unreacted
Stoichiometry Limiting Reagent Worksheet 2 Answers

Stoichiometry Limiting Reagent Worksheet 2 Answers
https://s3.studylib.net/store/data/025262652_1-f29aa5b335135028d153f5b57ca8edb7.png

Limiting Reagent Worksheet 1 Answers Chemistry Worksheets Practices
https://i.pinimg.com/originals/52/dd/4d/52dd4d3cd34dbca71302417c2846ac71.png

Limiting Reagent Worksheet 1
https://db-excel.com/wp-content/uploads/2019/09/limiting-reactants-worksheet-2-749x970.png
1 Determine the mass of lithium hydroxide produced when 0 38 g of lithium nitride reacts with water according to the following equation Li3N 3H2O gt NH3 3LiOH 2 What mass of sodium chloride is produced when chlorine reacts with 0 29 g of sodium iodide 3 Limiting Reagent Worksheet 2 1 Consider the reaction I2O5 g 5 CO g gt 5 CO2 g I2 g a 80 0 grams of iodine V oxide I2O5 reacts with 28 0 grams of carbon monoxide CO CO is limiting
Which is the limiting reagent Solution path 1 1 Calculate moles of sucrose 10 0 g 342 2948 g mol 0 0292146 mol 2 Calculate moles of oxygen required to react with moles of sucrose From the coefficients we see that 12 moles of oxygen are require for 3 What is the limiting reagent in the reaction described in problem 2 Because sodium iodide is the reagent that causes 8 51 grams of sodium nitrate to be formed it is the limiting reagent
More picture related to Stoichiometry Limiting Reagent Worksheet 2 Answers

Stoichiometry Limiting Reagent Worksheet Answers Db excel
https://db-excel.com/wp-content/uploads/2019/09/limiting-reagent-worksheet-1-4.png

Limiting Reagent And Percent Yield Worksheet
https://s3.studylib.net/store/data/008633156_1-38ac515997cae06d32815e09db3664f0.png

Limiting Reagents Worksheets
https://i.pinimg.com/originals/2d/e9/01/2de90177a1fa92a62eefd4b53a867c54.jpg
HW limiting reactant practice answers Chemistry Limiting Reactant Name Date 1 Mg O2 g 2 MgO What is the limiting reactant if 2 2 g of Mg is reacted with 4 5 L of oxygen at STP Both of Chemistry Stoichiometry Problem Sheet 2 Directions Solve each of the following problems Show your work including proper units to earn full credit CaCl2 AgNO3 Ca NO3 2 AgCl
Limiting Reagent Worksheet Solutions Using your knowledge of stoichiometry and limiting reagents answer the following questions 1 Write the balanced equation for the reaction of lead II nitrate with sodium iodide to form sodium nitrate and lead II iodide Pb NO3 2 aq 2 NaI aq 198 PbI2 s 2 NaNO3 aq Page 2 of 5 Limiting reagent problems for solution stoichiometry Now solve these 1 A solution of 100 0 mL of 0 200 M sodium chromate is mixed with a solution of 200 0 mL of 0 150 M strontium nitrate a What is the limiting reagent or reacting Sodium chromate b How many grams of precipitate form 0 293 g strontium chromate
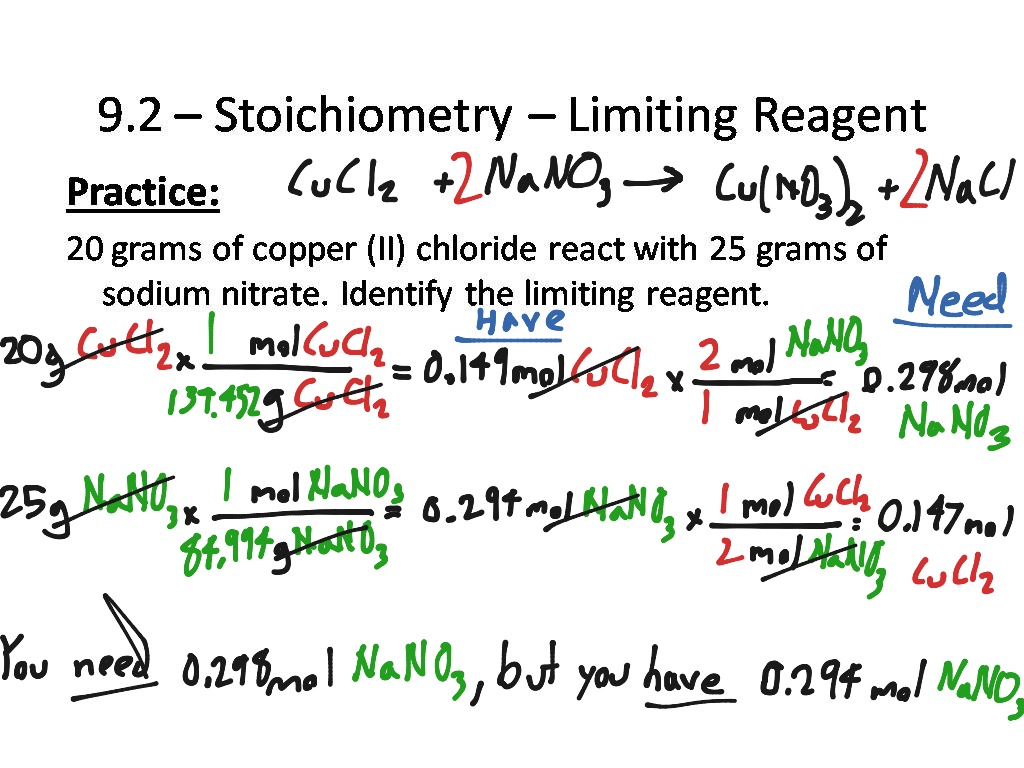
Stoichiometry Limiting Reagent Worksheet
https://showme0-9071.kxcdn.com/files/709559/pictures/thumbs/1876251/last_thumb1422513433.jpg

Limiting Reagent Worksheet Answer Key With Work Db excel
https://db-excel.com/wp-content/uploads/2019/09/limiting-reagent-worksheet-1-7-728x942.png
Stoichiometry Limiting Reagent Worksheet 2 Answers - 3 What is the limiting reagent in the reaction described in problem 2 Because sodium iodide is the reagent that causes 8 51 grams of sodium nitrate to be formed it is the limiting reagent