Which Does Not Increase The Speed Of Reaction Option A Adding a catalyst increases the speed of a reaction by providing an alternative pathway with a lower activation energy Option B Increasing the concentration of one of the reactants
Apr 10 2017 nbsp 0183 32 A catalyst is a substance that facilitates a reaction to take place at a fast rate then heat allows molecules to move fast so it increases the speed of reaction and increasing the The speed of chemical reactions DOES NOT increase with which of the following factors a increased temperature b increased concentration of reactants c increased particle size d
Which Does Not Increase The Speed Of Reaction

Which Does Not Increase The Speed Of Reaction
https://i.ytimg.com/vi/3CBSMb5kwdM/maxresdefault.jpg
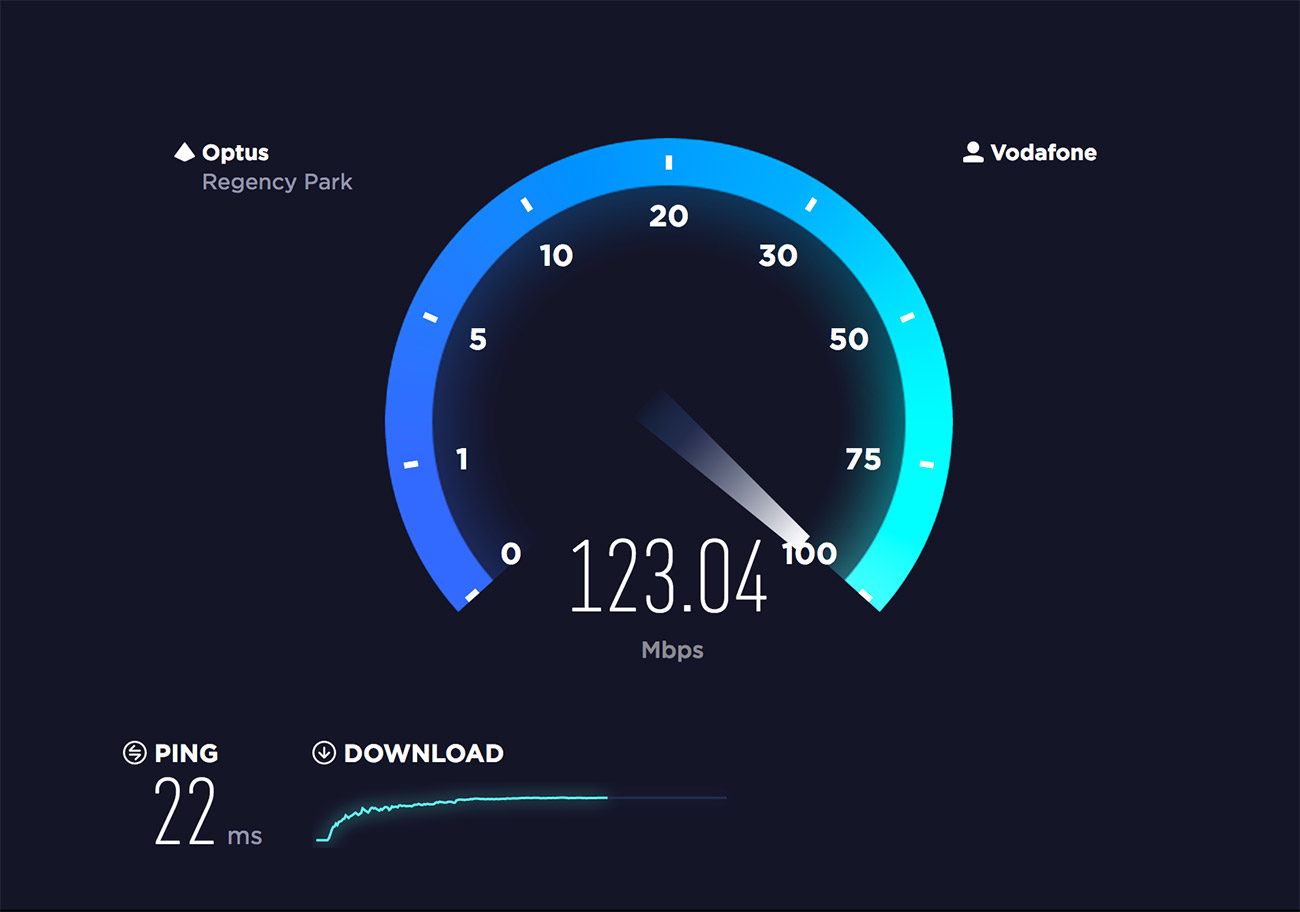
Speedtest Onlime
https://www.blogtyrant.com/wp-content/uploads/2017/02/speed_test.jpg

AulClear 2 CITCO Water
https://citcowater.com/wp-content/uploads/2023/12/ACD-Responsible-Distrubution-Award-2023.png
Jun 18 2020 nbsp 0183 32 What does not increase the speed of a reaction Answer If the reactants don t have enough energy a reaction won t occur even if the reactants do collide at the reactive site Mar 13 2021 nbsp 0183 32 When solid zinc is dropped into hydrochloric acid solution it falls to the bottom due to its higher density The reaction occurs at the surface of the zinc metal and generates hydrogen gas that bubbles up through the solution You
Which of the following does not influence the speed of a chemical reaction A time vs concentration graph is presented below for the reaction A B What is the rate of appearance Jul 14 2022 nbsp 0183 32 The factor that does not increase the rate of a reaction is A Decreasing the concentration of the reactants In contrast increasing temperature surface area or adding a
More picture related to Which Does Not Increase The Speed Of Reaction

Earring Flower Flor Pendant By Danae Download Free STL Model
https://media.printables.com/media/prints/790053/images/6124223_ea90ba19-abd3-4ec8-813b-96a40d2a8237_8be3f351-bc04-4a64-b734-eeb8a36e12e4/photo1709430907.jpeg

Easy Stuffing Balls Recipe From Val s Kitchen
https://www.fromvalskitchen.com/wp-content/uploads/2023/07/mkia-fvk-new-logo.png

Best Stretches For Erectile Dysfunction Discount Laseb fae ufmg br
https://media.zesttee.com/cms/6-exercises-to-increase-penis-blood-flow_9665-a.jpg
May 3 2023 nbsp 0183 32 The factor that does not increase the rate of a reaction is C decreasing the concentration of the reactants Lower concentration leads to fewer collisions and a slower Oct 29 2020 nbsp 0183 32 For example increasing temperature speeds up a reaction but above a certain temperature the reactants may denature Adding a catalyst speeds a reaction but adding more of it won t cause a further rate increase
We use a burner or a hot plate in the laboratory to increase the speed of reactions that proceed slowly at ordinary temperatures In many cases an increase in temperature of only 10 176 C will This tells us that step 2 occurs faster than step 1 So the concentration of intermediate should not increase over the course of the reaction and it should react at least as fast as it is formed The

Determine The Probability Of The Treatment Group s Mean Being Lower
https://us-static.z-dn.net/files/d21/7212f18564f2d1f5b710a17c35b23a29.png
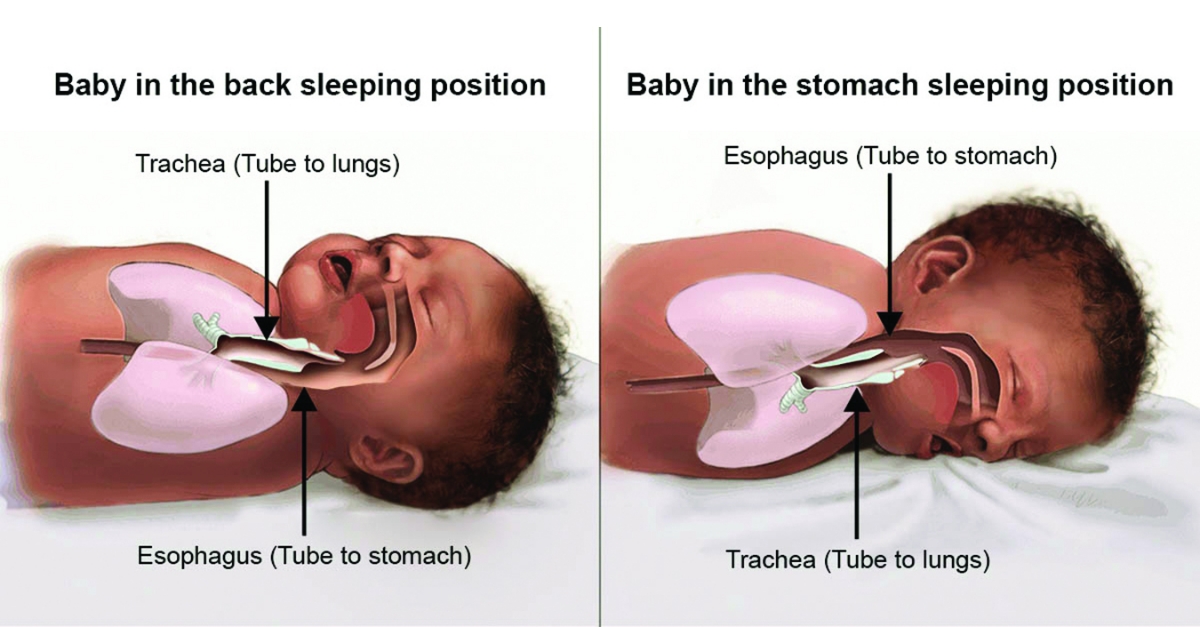
About Back Sleeping Safe To Sleep
https://safetosleep.nichd.nih.gov/sites/default/files/inline-images/SleepPositionIllustration-1600x900.jpg
Which Does Not Increase The Speed Of Reaction - Nov 8 2024 nbsp 0183 32 Increasing temperature concentration and using a catalyst all speed up the reaction rate However using larger pieces of a solid reagent does not lead to an increase in