Naming And Covalent Compounds Worksheet Answers Naming Ionic Compounds Practice Worksheet Name the following ionic compounds 1 NH 4 Ionic Covalent Compound Naming Solutions 1 Na 2 CO 3 sodium carbonate 2 P 2 O 5 diphosphorus pentoxide 3 NH 3 More Naming Practice Answers 1 BBr 3 boron tribromide 2 CaSO 4 calcium sulfate 3 C 2 Br 6
7 ammonia nitrogen trihydride NH3 8 phosphorus triiodide PI3 9 dihydrogen monoxide H20 10 diphosphorous pentoxide P2O5 Write the names for the following covalent compounds 11 P4S5 tetraphosphorus pentasulfide 12 O2 dioxide oxygen 13 SeF6 selenium hexafluoride 14 Si2Br6 disilicon hexabromide Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class education for anyone anywhere
Naming And Covalent Compounds Worksheet Answers
Naming And Covalent Compounds Worksheet Answers
http://2.bp.blogspot.com/-eAfnIDsTasU/Tyf2Xi-aiPI/AAAAAAAAA0g/q4XkrfTiPNw/s1600/Naming%2BAcids%2BWS.JPG

11 Best Images Of Ionic And Covalent Bonding Practice Worksheet Answers
http://www.worksheeto.com/postpic/2015/03/naming-ionic-and-covalent-compounds-worksheet_209448.png

Tom Schoderbek Chemistry December 2014
https://lh5.googleusercontent.com/-PCdVswb2b6A/VITv5kI2VHI/AAAAAAAAAQM/uk3TPftzbEA/s640/blogger-image--297102716.jpg
Covalent compound naming Covalent Compound Naming Practice For those of you who love covalent compounds you re in for a real treat More Covalent Compound Naming Practice And if you still love covalent compounds even after the last worksheet here s another for your viewing pleasure Naming Covalent Compounds dd ch Let s name our Write the formulas of the following acids and bases 99 hydrobromic acid HBr 100 hydrofluoric acid HF 101 carbonic acid H2CO3 102 lithium hydroxide LiOH 103 nitrous acid HNO2 104 cobalt II hydroxide Co OH 2 105 sulfuric acid H2SO4 106 beryllium hydroxide Be OH 2
Bonding interactions and naming compounds 8 1 molecular compounds the octet rule in covalent Bonding Covalent compounds are most stable when each atom has eight electrons Single double and triple covalent bonds depend on the number of pairs of electrons after reading Lesson 8 3 answer the following questions molecular orbitals 1 Why are ionic compounds so easy to name Because most ionic compounds can only form one way using the oxidation numbers In covalent compounds though non metals can sometimes com bine in multiple ways carbon monoxide carbon dioxide So covalent compounds use prefixes Hints to remember prefixes Mono rail one rail train
More picture related to Naming And Covalent Compounds Worksheet Answers

Naming Compounds With Transition Metals Worksheet Answers Printable
https://i.pinimg.com/originals/0a/2b/a9/0a2ba9fc09004838eacdd0f231c0b3ca.jpg

Chemical Bonding Worksheet With Answers Pdf
https://i.pinimg.com/originals/38/bd/b6/38bdb69a48865aae4bee5797b1d3ed24.png

Covalent Compounds Worksheet Formula Writing And Naming Worksheet
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/02/covalent-compounds-worksheet-formula-writing-and-naming.jpg
Covalent Compound Naming Worksheet 1 covalentname sxw Naming Covalent Compounds Solutions Write the formulas for the following covalent compounds 1 antimony tribromide SbBr3 2 hexaboron silicide B6Si 3 chlorine dioxide ClO2 4 hydrogen iodide HI 5 iodine pentafluoride IF5 COVALENT BONDING Name Covalent bonding occurs when two or more nonmetals share electrons attempting to attain a stable octet of electrons at least part of the time For example Note that hydrogen Is content with 2 not 8 electrons Show how covalent bonding occurs in each of the following pairs of atoms Atoms may
Here are the rules for naming binary covalent compounds A binary compound is one that consists of only two elements The names are called systematic names First name the nonmetal furthest to the left and bottom of the periodic table by its element name Second name the other nonmetal by its element name but shorten its name and add an ide Ionic and Covalent Compounds Name KEY 1 We differentiate between two types of compounds IONIC and COVALENT 2 C12H22O11 is MOLECULAR or COVALENT compound while sodium chloride table salt is an IONIC compound 6 Carbon monoxide CO is an example of a diatomic molecule while ammonia and glucose NH3 and C6H12O6 are examples

Covalent Compounds Worksheets Formula Writing And Naming Answers
https://www.housview.com/wp-content/uploads/2018/07/naming_covalent_compounds_worksheet_answer_sheet_831228_4.jpg
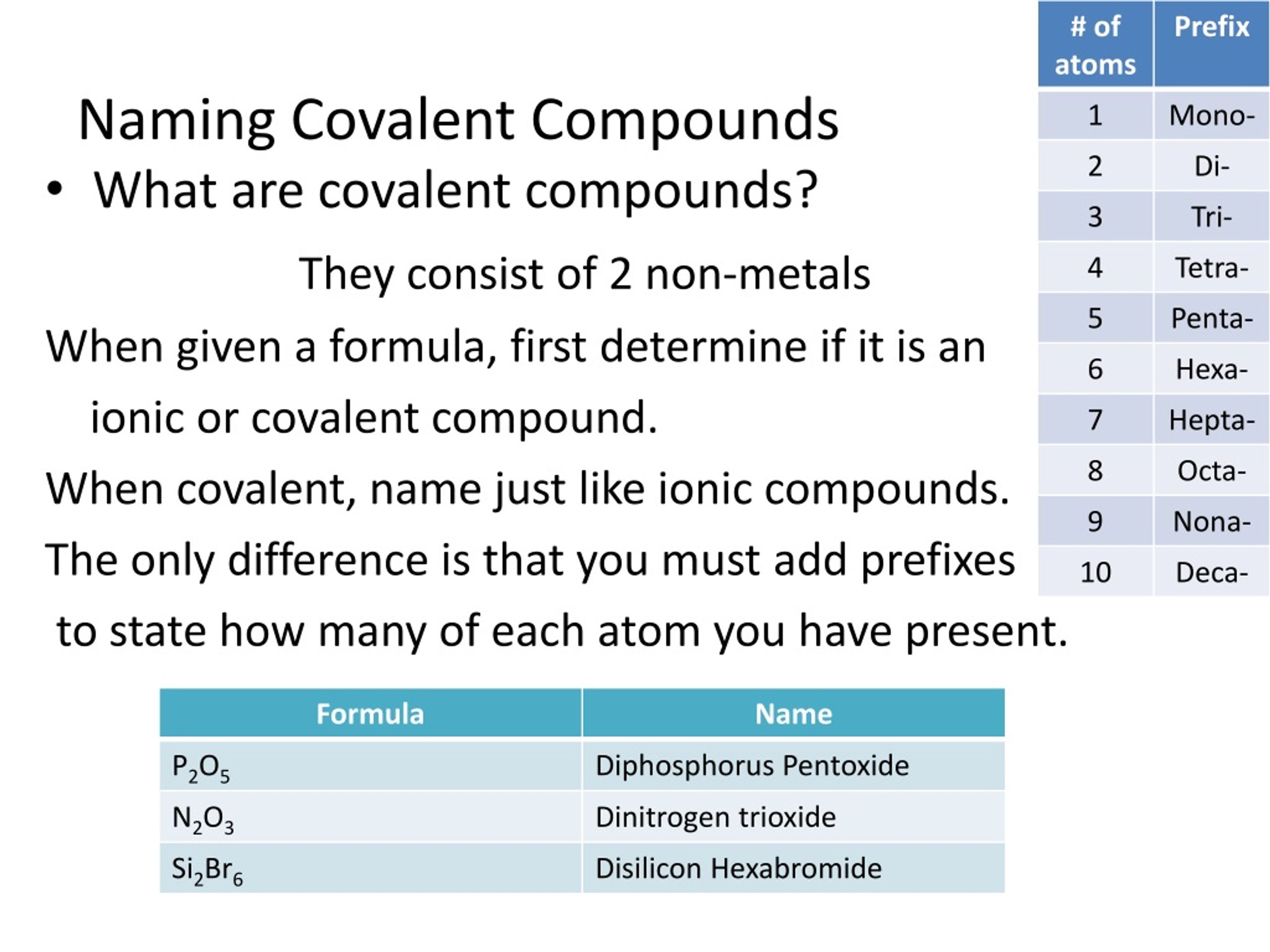
PPT Covalent Compounds And Naming PowerPoint Presentation Free
https://image4.slideserve.com/455065/naming-covalent-compounds-l.jpg
Naming And Covalent Compounds Worksheet Answers - Created Date 11 12 2013 5 43 06 PM