Empirical And Molecular Formula Worksheet Answer Key Created Date 2 9 2018 11 35 42 AM
Empirical and Molecular Formulas Worksheet 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O 3 What is empirical formula if compound consists of 21 2 N 6 1 H 24 2 S and 48 5 O 5 Determine the empirical and molecular formula of a compound composed of 18 24 g Carbon ol Empirical Formula Molecular Formula 6 A compound with a molar mass of 544 0 g mol s made up of 26 5 grams Carbon 2 94 grams H Empirical Formula Molecular Formula
Empirical And Molecular Formula Worksheet Answer Key

Empirical And Molecular Formula Worksheet Answer Key
https://data.formsbank.com/pdf_docs_html/88/884/88467/page_1_thumb_big.png

Empirical And Molecular Formula Practice Solutions Docsity
https://static.docsity.com/documents_first_pages/2021/04/20/f27b8372eab457d749d70fed0e1dc877.png?v=1646976324

Empirical And Molecular Formula Worksheet Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/ch10-empirical-and-molecular-formula-worksheet-768x994.png
Created Date 20180411075110Z This activity will compare two types of useful formulas Model 1 Comparison of Percent Composition and Empirical Formula Substance Compound Name Molecular Formula Empirical formula Percent Composition Carbon Percent Composition Hydrogen
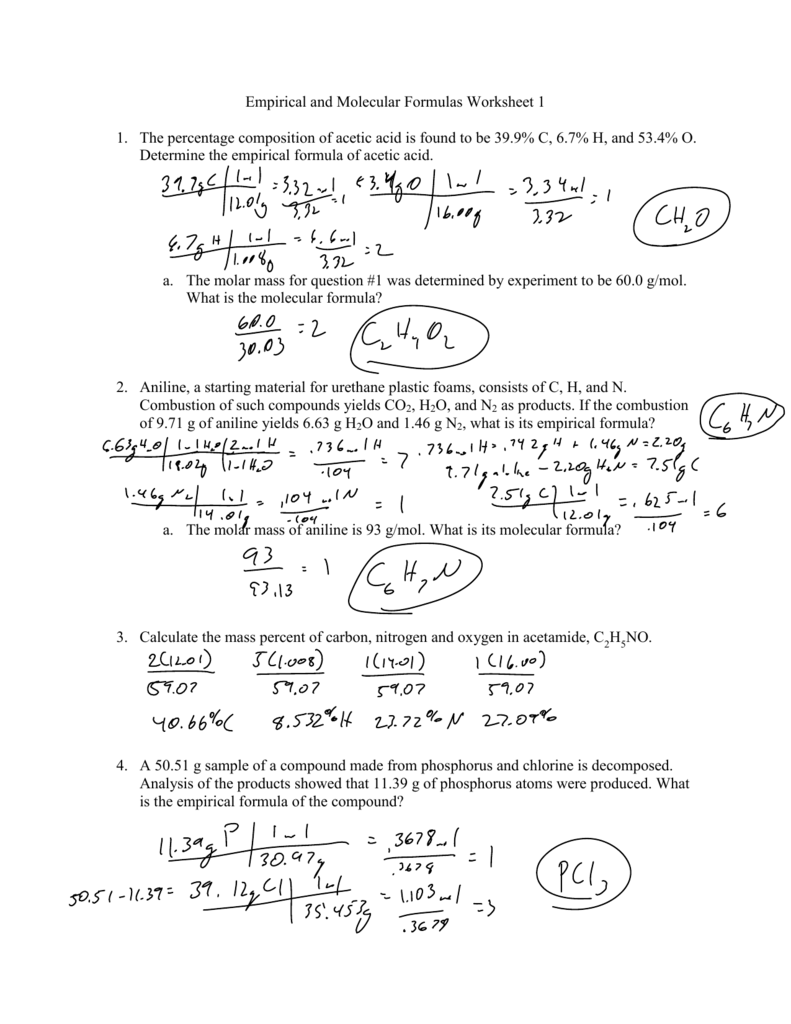
Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in the problems are grams Step 1 convert to moles Step 2 divide each by the lowest number of moles Step 3 only if necessary multiply all by the same factor in order to obtain whole numbers Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds 1 C6H6
More picture related to Empirical And Molecular Formula Worksheet Answer Key
Practice Quiz Empirical And Molecular Formula Empirical And Molecular
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/591469cf9c1b7c8839f52e3ebf6a3459/thumb_1200_1835.png

Empirical And Molecular Formula Worksheet Answer Key
https://briefencounters.ca/wp-content/uploads/2018/11/empirical-and-molecular-formula-worksheet-answer-key-with-empirical-and-molecular-formula-worksheet-answers-lukaspesa-of-empirical-and-molecular-formula-worksheet-answer-key.jpg
Empirical Formula Worksheets Key
https://www.unmisravle.com/wp-content/uploads/2018/04/empirical_and_molecular_formula_worksheet_2.png
EMPIRICAL FORMULAS To determine the empirical formula of a compound 1 Determine the relative weights of the elements that make up the compound if they have not already been provided 2 Express these quantities in moles 3 Divide the number of moles by the minimum number of moles for each element 4 Create a ratio for the elements in the The empirical formula of a compound is known to be CH2 and its molar mass is 56 g mol What is the molecular formula 12 A compound contains 12 8 C 2 1 H and 85 1 Br by mass Calculate the empirical formula and the molecular formula of this compound given that the molar mass is 188 g mol 12 8 BC 12 0131m quot 1 85 1q gr 1 019 13
Download Study notes Empirical and Molecular Formula Worksheet ANSWER KEY Brussels School of International Studies A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole Determine the empirical formulas for compounds with the following percent compositions a 15 8 carbon and 84 2 sulfur b 40 0 carbon 6 7 hydrogen and 53 3 oxygen Answer a Answer b Click here to see a video of the solution

Empirical molecular Formula Worksheets Answer Key
https://www.unmisravle.com/wp-content/uploads/2018/03/percent_composition_and_molecular_formula_worksheet_1.jpg

Empirical And Molecular Formula Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/empirical-and-molecular-formula-worksheet-768x994.png
Empirical And Molecular Formula Worksheet Answer Key - Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in the problems are grams Step 1 convert to moles Step 2 divide each by the lowest number of moles Step 3 only if necessary multiply all by the same factor in order to obtain whole numbers