Empirical And Molecular Formula Worksheet Answers Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula
Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula 2 Multiply all the subscripts in the empirical formula by the answer to the previous step Example 2 If the compound from Example 1 had a molecular weight of 306 g what would the molecular formula be Solution The empirical formula was A 2 O 3 The empirical formula weight is 2 27 0 g 3 16 0 g 102 g
Empirical And Molecular Formula Worksheet Answers

Empirical And Molecular Formula Worksheet Answers
https://www.problemsworksheets.com/wp-content/uploads/2023/03/empirical-formula-worksheet-answers-free-download-qstion-co.jpg
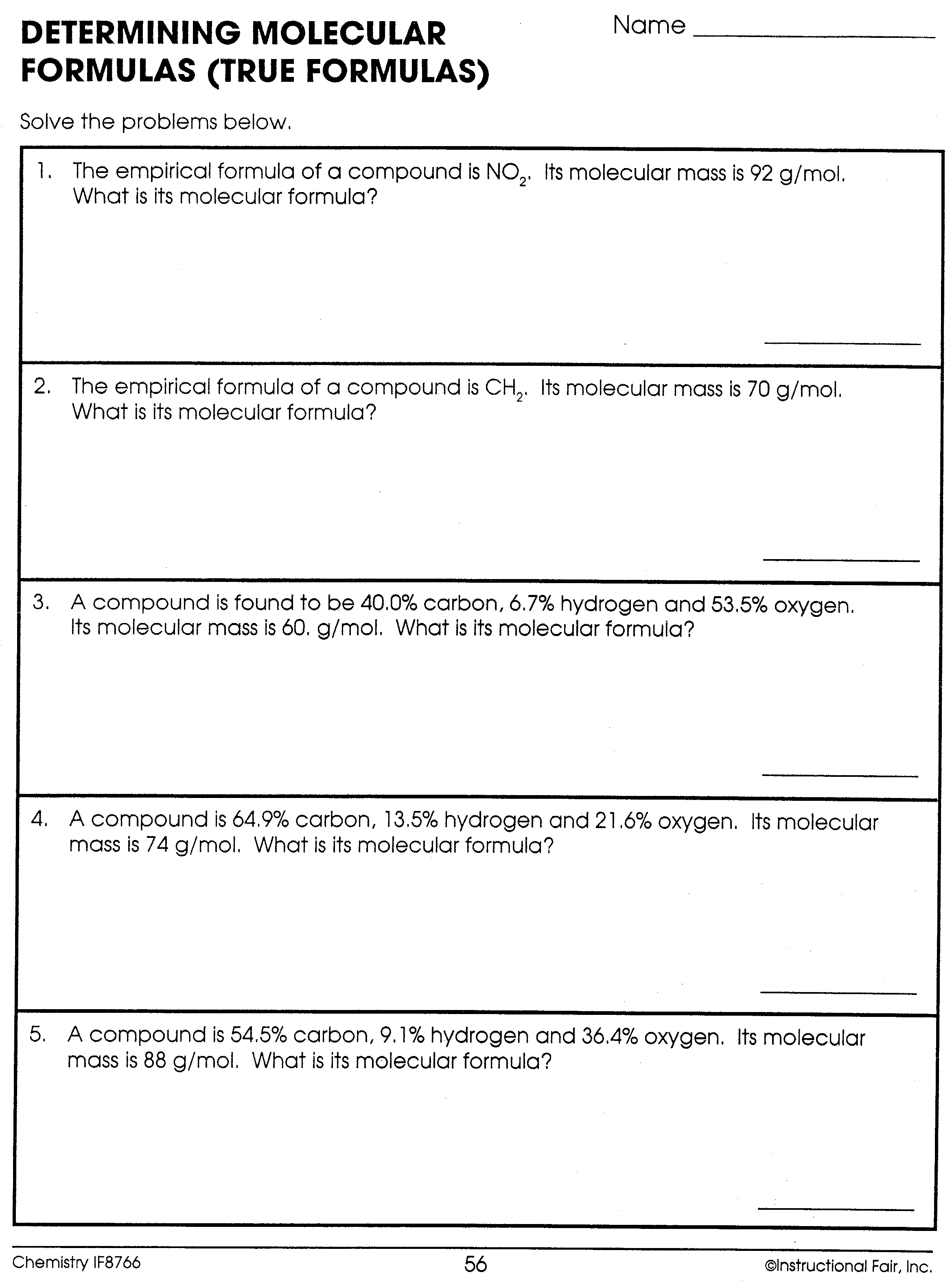
Empirical And Molecular Formula Problems Pdf UPDATED
http://66.39.52.159/ddavis/CHif56.bmp

Free Printable Percent Composition And Empirical Formula Worksheets
https://www.chemistrylearner.com/wp-content/uploads/2023/05/Percent-Composition-and-Empirical-Formula-Worksheet-with-Answer-Key.webp
Determine the molecular formula for each compound whose percentage composition is shown below 6 84 9 Hg and the remainder Cl with a molecular weight of 472 2 g mol Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in 16 The empirical formula of garnet a gemstone is Fe 3 Al 2 Si 3 O 12 An analysis of a sample of garnet gave a value of 13 8 for the mass percentage of silicon Is this consistent with the empirical formula No the calculated mass percentage of silicon in garnet is 16 93 17 C 4 H 8 O 2 18 Empirical Formula C 10 Cl 12 Molecular
Show your work for the calculation of empirical formula here Excessive physical activity lactic acid molecular mass 90 08 g per mole forms in muscle tissues and is responsible for muscle soreness Elemental analysis shows that this compound has 40 0 carbon 6 71 hydrogen and 53 3 oxygen Determine the empirical formula of lactic acid Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER Write the empirical formula for the following compounds 1 C6H6 2 C8H18 3 WO2 Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds 1 C6H6 C 3 H 3 6 C8H18 C 4 H 9 7 WO2 WO 2 8 C2H6O2 CH 3 O
More picture related to Empirical And Molecular Formula Worksheet Answers

Empirical And Molecular Formula Worksheet ANSWER KEY Study Notes
https://static.docsity.com/documents_first_pages/2022/08/05/277f9018d3a6f7b945d95b256c059b89.png?v=1672948397

Difference Between Empirical And Molecular Formula Infographic
https://i.pinimg.com/originals/d9/5a/e2/d95ae2a472e78cb40f5689c929df31af.jpg

Percent Composition And Molecular Formula Worksheet
https://s3.studylib.net/store/data/007461692_1-1d0e0c491fb7c25b1c9f40e799b3b1a6.png
Empirical and Molecular Formulas Worksheet 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O 3 What is empirical formula if compound consists of 21 2 N 6 1 H 24 2 S and 48 5 O The ratio is 1 000 mol of iron to 1 500 mol of oxygen Fe 1 O 1 5 Finally multiply the ratio by two to get the smallest possible whole number subscripts while still maintaining the correct iron to oxygen ratio 2 Fe1O1 5 Fe2O3 2 Fe 1 O 1 5 Fe 2 O 3 The empirical formula is Fe 2 O 3
The molecular formula of a compound is defined as the actual number of atoms of elements covalently bonded in a molecule and is a whole number ratio of the empirical formula The percentage composition of a compound can be determined from its formula or from experimental mass proportion data Titanium oxide TiO2 is widely used as a pigment Step 1 Calculate the molar mass of the empirical formula empirical mass Step 2 Divide the actual formula mass by the empirical mass Step 3 Multiply the subscripts in the empirical formula by the answer in Step 2 Example The empirical formula for vitamin C is C3H4O3 Experimental data indicates that the molecular mass of vitamin C is

30 Awesome Molar Mass Chem Worksheet 11 2 Answer Key Worksheet And
https://i.pinimg.com/736x/eb/a3/d7/eba3d7451df51dffbb3eb18cac1869d9.jpg

Chemistry Empirical And Molecular Formula Worksheet Answers
https://www.chemistryworksheet.com/wp-content/uploads/2022/10/empirical-and-molecular-formula-worksheets-answers.jpg
Empirical And Molecular Formula Worksheet Answers - Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound 7 A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole