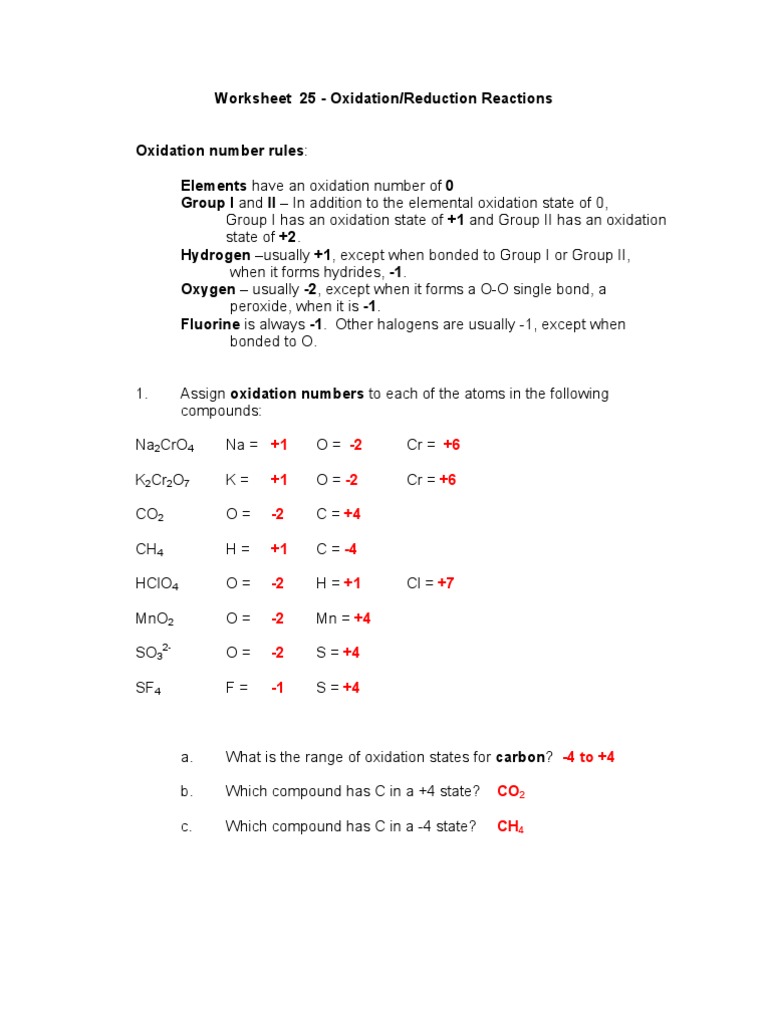
Assigning Oxidation Numbers Worksheet Answer Key WORKSHEET ASSIGNING OXIDATION NUMBERS Name Period Oxidation Number Rules A pure element has an oxidation number of 0 The oxidation number of an element in a monatomic ion equals the charge of the ion The sum of the oxidation number of all the elements in a compound equals 0
Balance the following and calculate the change in oxidation number for the species oxidized or reduced Compare this to the number of electrons in the balanced reaction Apr 7 2015 nbsp 0183 32 Purpose This exercise is designed to teach the student how to assign oxidation numbers Oxidation numbers are very important and are used for 1 naming compounds 2 balancing oxidation reduction reactions 3 calculations in electrochemistry and other areas of chemistry Exercises Give the oxidation number for the following atoms 2 0 F
Assigning Oxidation Numbers Worksheet Answer Key

Assigning Oxidation Numbers Worksheet Answer Key
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/04/what-are-oxidation-numbers-worksheet.jpg

Assigning Oxidation Numbers Worksheet Answer Key Worksheet Resume
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/02/assigning-oxidation-numbers-worksheet-and-answers.jpg
Oxidation State Worksheets
http://www.unmisravle.com/wp-content/uploads/2018/03/pictures_oxidation_number_practice_worksheet_1.jpg
Oxidation Number Rules 1 A pure element has an oxidation number of 0 2 The oxidation number of an element in a monatomic ion equals the charge of the ion 3 The sum of the oxidation number of all the elements in a compound equals 0 4 The sum of the oxidation numbers of all the elements in a polyatomic ion equals the charge on the ion Rules for Assigning Oxidation Numbers The oxidation number of any uncombined element is 0 The oxidation number of a monatomic ion equals the charge on the ion The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion
Rules for assigning oxidation numbers The oxidation number of an element is zero For a monatomic ion the oxidation number is the charge on the ion The oxidation number of combined hydrogen is usually 1 The oxidation number of combined oxygen is usually 2 Rules for Assigning Oxidation Numbers 1 The oxidation number of any uncombined element is 0 2 The oxidation number of a monatomic ion equals the charge on the ion 3 The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion 4
More picture related to Assigning Oxidation Numbers Worksheet Answer Key

Oxidation QXCH
https://i0.wp.com/s1.studyres.com/store/data/017958765_1-22f5892455c3cabe89ebd5bb7d32dc7c.png

Rules For Assigning Oxidation Numbers
https://s3.studylib.net/store/data/008716718_1-18f0577b8e071d7025c67ff74334772c.png
Charting Oxidation Number Worksheet Answer Key Worksheets Joy
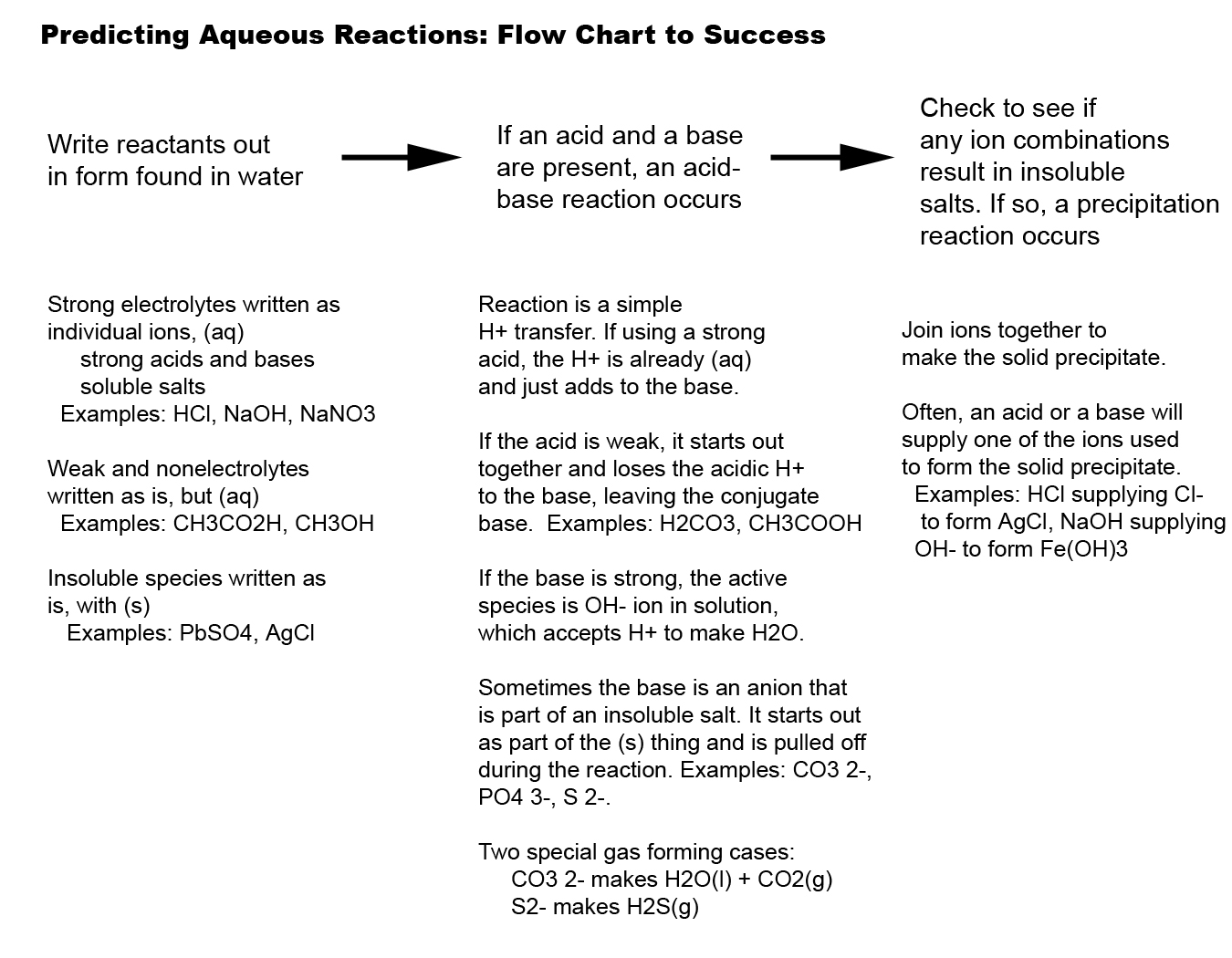
http://employees.oneonta.edu/viningwj/Chem111/Predicting Aqueous Reactions.jpg
OXIDATION NUMBER The charge which an atom has or appears to have when electrons are counted according to certain arbitrary rules In assigning oxidation numbers electrons shared between two unlike atoms are counted as belonging to the more electronegative atom WORKSHEET ASSIGNING OXIDATION NUMBERS Name Period Oxidation Number Rules A pure element has an oxidation number of 0 The oxidation number of an element in a monatomic ion equals the charge of the ion The sum of the oxidation number of all the elements in a compound equals 0
Mar 13 2023 nbsp 0183 32 Understand the rules for assigning oxidation numbers Understand the concepts of oxidation and reduction in terms of oxidation numbers Understand solution concentration in terms of molarity Know the connection between molarity volume The oxidation state of an atom is represented by a positive or negative number called its oxidation number Chemists have developed rules used to assign oxidation numbers The following rules will help you determine the oxidation state of an atom or ion A free atom has an oxidation number of zero It is not sharing gaining or losing electrons

Worksheet Oxidation Numbers Answer Key Ivuyteq
http://www.fabtemplatez.com/wp-content/uploads/2018/03/worksheet-oxidation-numbers-answers-45240-chemistry-iiib-mr-phelps-big-rapids-hs-worksheet-oxidation-numbers-answers-17551275.jpg

Oxidation Reduction Worksheet Answers
https://media.cheggcdn.com/media/874/87475515-ed75-4017-bf20-b8879396dc53/phpePYrdh.png
Assigning Oxidation Numbers Worksheet Answer Key - Rules for Assigning Oxidation Numbers 1 The oxidation number of any uncombined element is 0 2 The oxidation number of a monatomic ion equals the charge on the ion 3 The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion 4