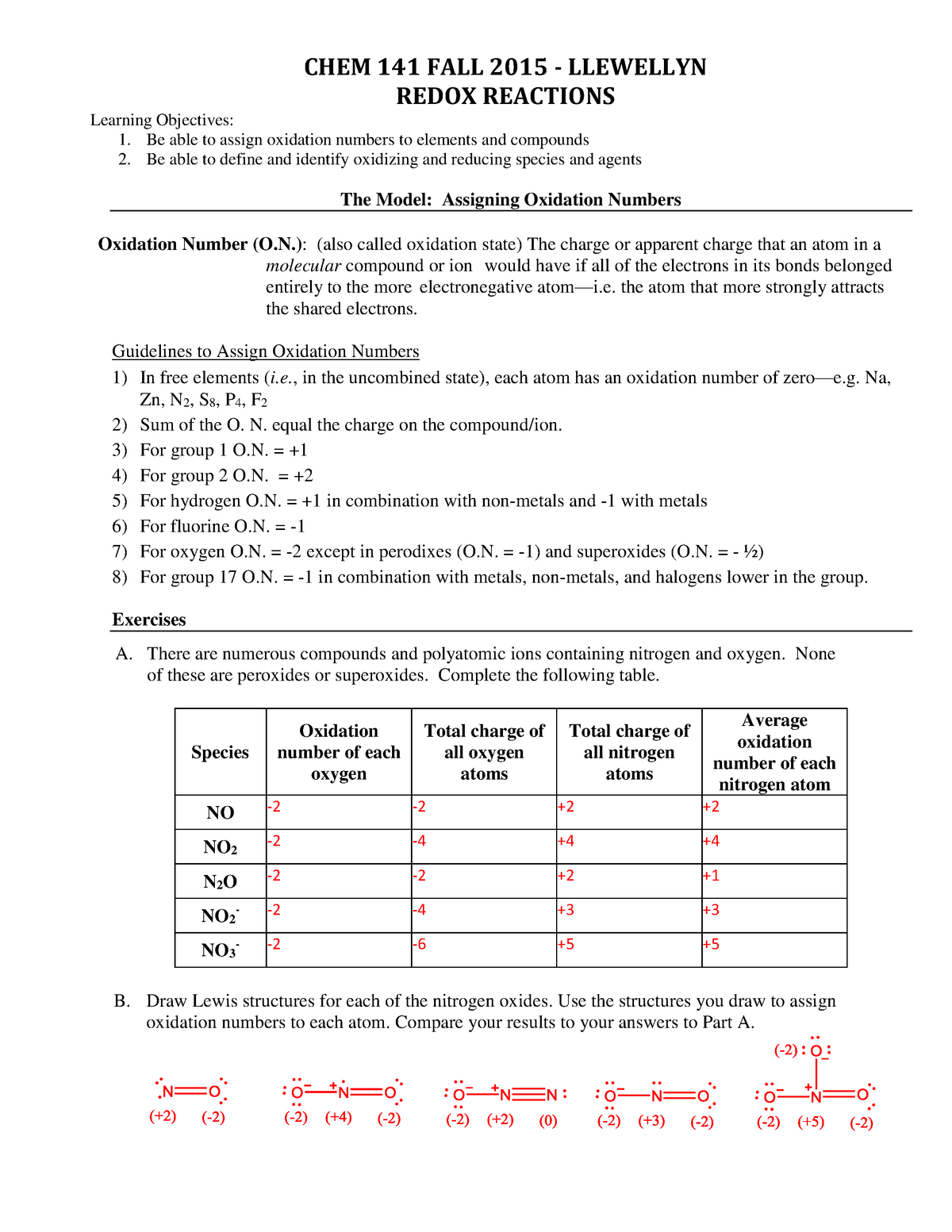
Worksheet Oxidation Numbers Answer Key Rule 1 The oxidation number of an element in its free uncombined state is zero for example Al s or Zn s This is also true for elements found in nature as diatomic two atom elements H2 O2 S8 Rule 2 The oxidation number of a monatomic one atom ion is the same as the charge on the ion for example Na S2
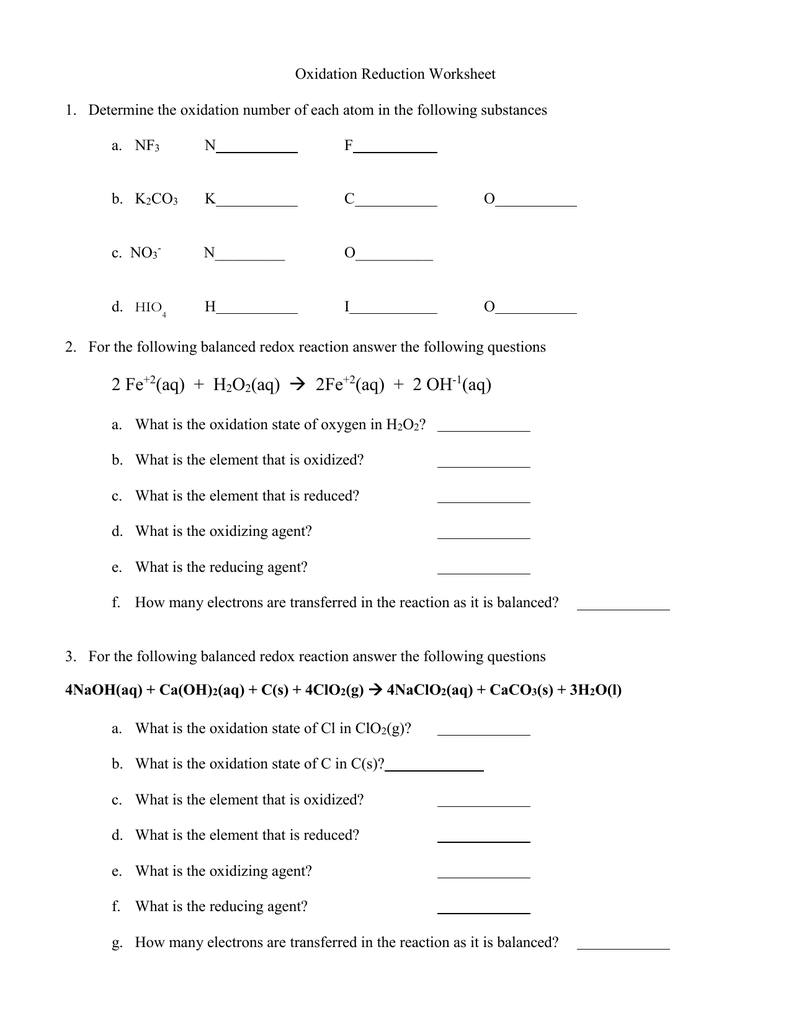
Use the changes in oxidation numbers to determine which elements are oxidized and which are reduced in these reactions Note it is not necessary to use balanced equations C H2SO4 HNO3 HI KMnO4 HCl Sb HNO3 CO2 SO2 H2O NO I2 H2O MnCl2 Cl2 H2O KCl Sb2O3 NO H2O Q1 Show the electron configuration for the transition metal cation using the box notation in the table Indicate if the complex is paramagnetic or not in the final column of the table Complete the oxidation number column of the table below by working out the oxidation number of each of the transition metal cations
Worksheet Oxidation Numbers Answer Key
Worksheet Oxidation Numbers Answer Key
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/4bc9486e8f803cab8ab45811749b2b2d/thumb_1200_1553.png

Worksheet Oxidation Numbers Answers
http://www.fabtemplatez.com/wp-content/uploads/2018/03/oxidation-numbers-worksheet-answers-57606-5-1-oxidation-numbers-oxidation-numbers-worksheet-answers-1024791.jpg
Charting Oxidation Number Worksheet Answer Key Worksheets Joy
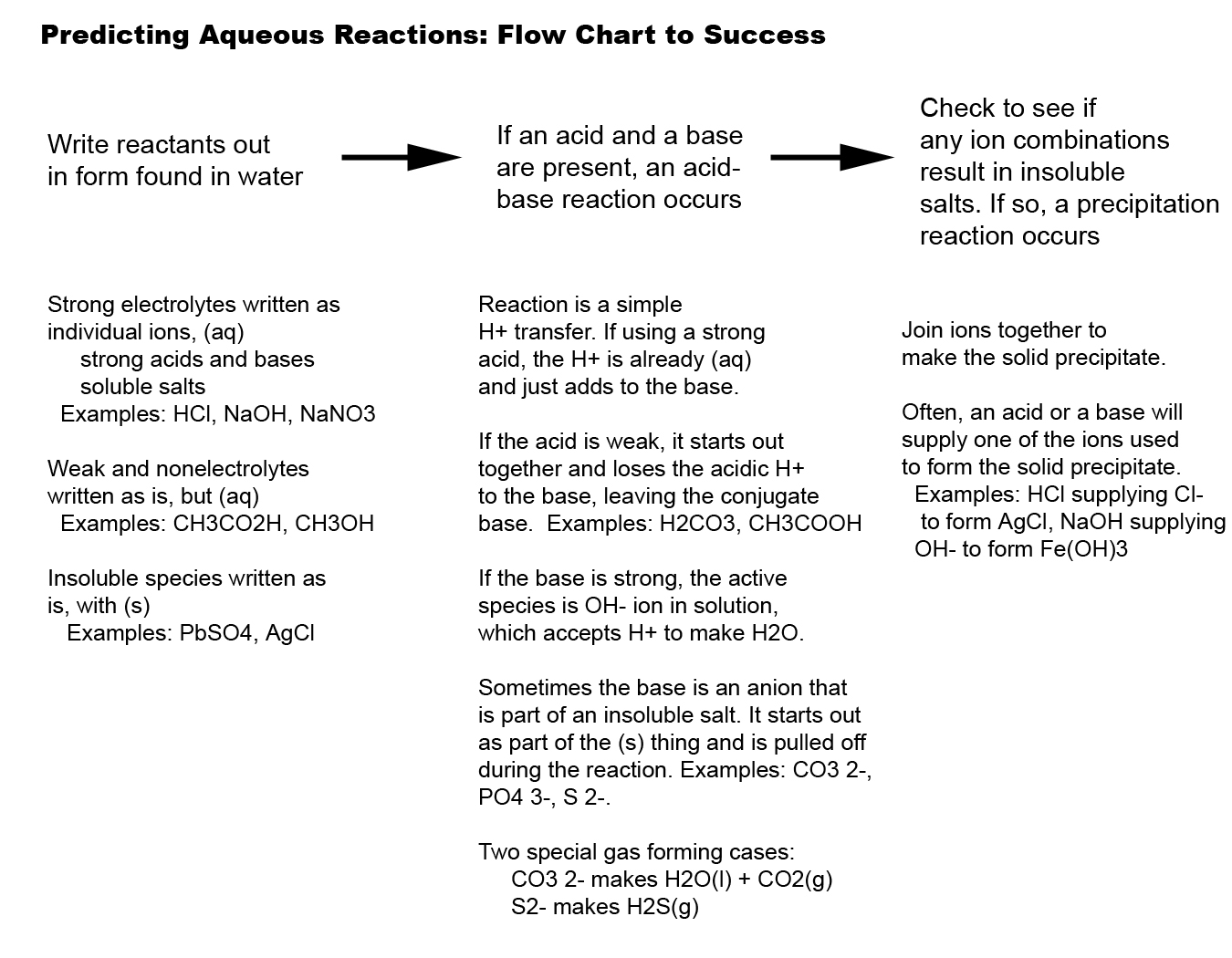
http://employees.oneonta.edu/viningwj/Chem111/Predicting Aqueous Reactions.jpg
A monoatomic ion has an oxidation number equal to its charge For example the oxidation number of the oxygen in the oxide ion O 2 is 2 The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion Let s examine the oxidation numbers of some common elements Notice the periodic trend among the main group Worksheet Assigning Oxidation Numbers Key Oxidation answers Course Earth Science EAR 105 112Documents Students shared 112 documents in this course University Syracuse University Academic year 2016 2017 Uploaded by Anonymous Student This document has been uploaded by a student just like you who decided to remain anonymous
Show the oxidation state changes for each element a b c 9 Compare the oxidation state of hydrogen in HCl with its oxidation state in NaH Why are they different 10 Describe the trend in oxidation number for main group elements as you move left to right across a row in the periodic table 11 The oxidation number of Al subtract four equals 1 Therefore the oxidation number of Al is 3 3 Work out the oxidation numbers for the elements in the following molecules by first assigning and charges a NBr 3 b BeCl 2 c BI 3 d CH 3 OH e HO f H 2 PO 4 You should now learn the rules for assigning oxidation numbers
More picture related to Worksheet Oxidation Numbers Answer Key

Assigning Oxidation Numbers Worksheet And Answers Worksheet Resume
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/04/worksheet-oxidation-numbers-answers.jpg
Oxidation Reduction Worksheet Answers
https://s2.studylib.net/store/data/015301580_1-e092699c030435ee2c166eccfa76c1db.png
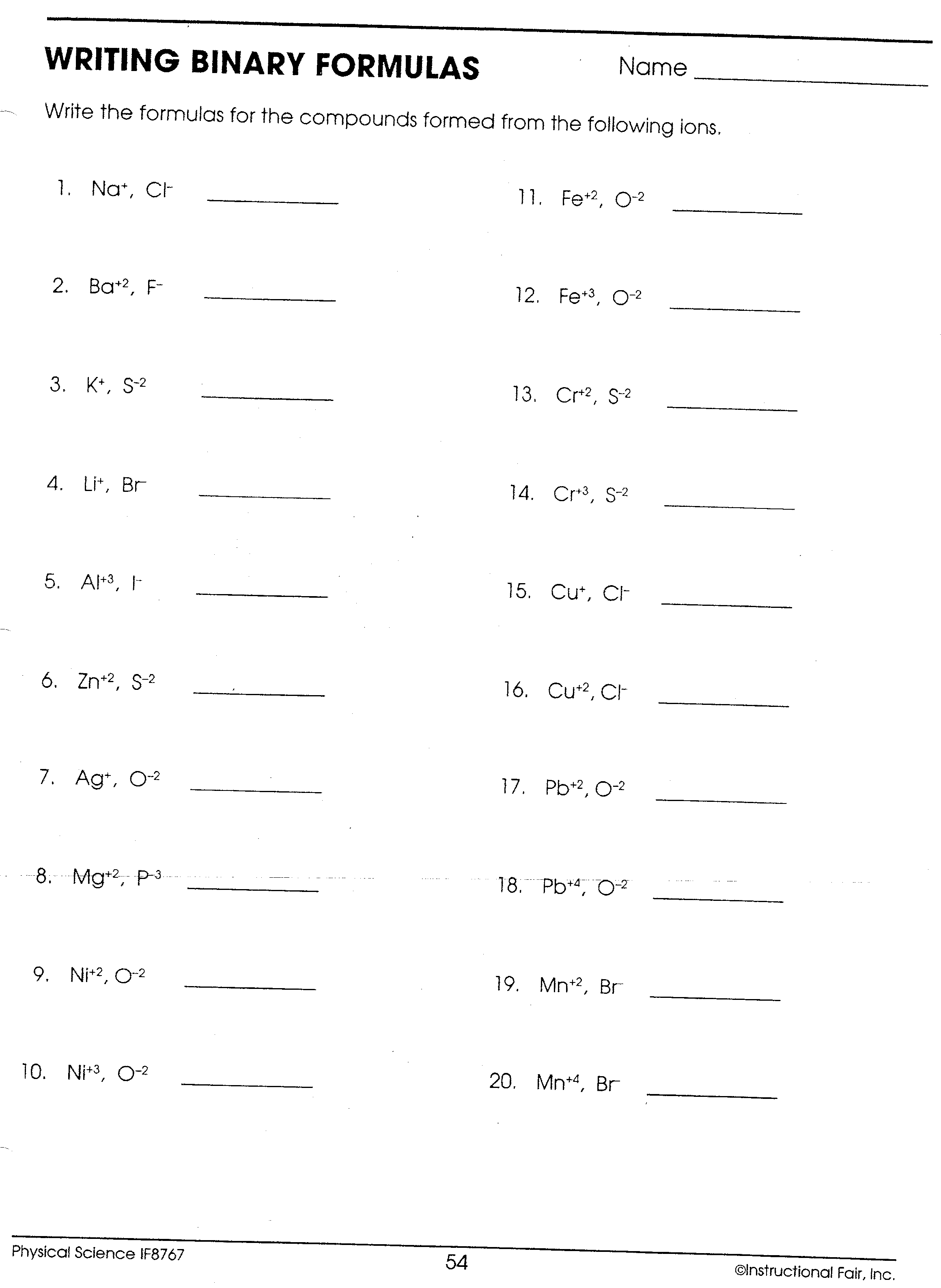
Worksheet Oxidation Numbers Worksheet Grass Fedjp Worksheet Study Site
http://66.39.52.159/ddavis/PSif54.bmp
Worksheet 1 Name Period Seat Directions Use the rules for Assigning Oxidation numbers to determine the oxidation number assigned to each element in each of the given formulas The rules are at the bottom of the page Rules for Assigning Oxidation Numbers The oxidation number of any uncombined element is 0 With this Colour by Number worksheet chemistry or science students can practice calculating oxidation numbers oxidation states as part of the Oxidation Reduction RedOx Unit in a fun and relaxing way which also improves memory Students will first calculate the oxidation numbers of the underlined elements in the different compounds
Chem 115 POGIL Worksheet Week 6 Answers Oxidation Numbers Redox Reactions Solution Concentration Titrations First Law and Enthalpy Key Questions Exercises and Problems 1 Assign the oxidation numbers of each element in the following chemical species HCl H2O NH 3 NO 3 K 2Cr 2O 7 Hg2Cl 2 HgCl 2 Al OH 3 Na3PO 4 1 1 HCl Write balanced equations for the following redox reactions a 2 NaBr Cl 2 2 NaCl Br 2 b Fe 2 O 3 3 CO 2 Fe 3 CO 2 in acidic solution c 5 CO I 2 O 5 5 CO 2 I 2 in basic solution a Cr OH 3 Br 2 CrO 42 Br in basic solution 10 OH 2 Cr OH 3 3 Br 2 2 CrO 42 8 H 2 O 6 Br b

Oxidation Number Worksheet 1 Write The Rule Next To Your Answer
https://s3.studylib.net/store/data/008852526_1-284d9339a86fac54ce8d6274bceb7050.png
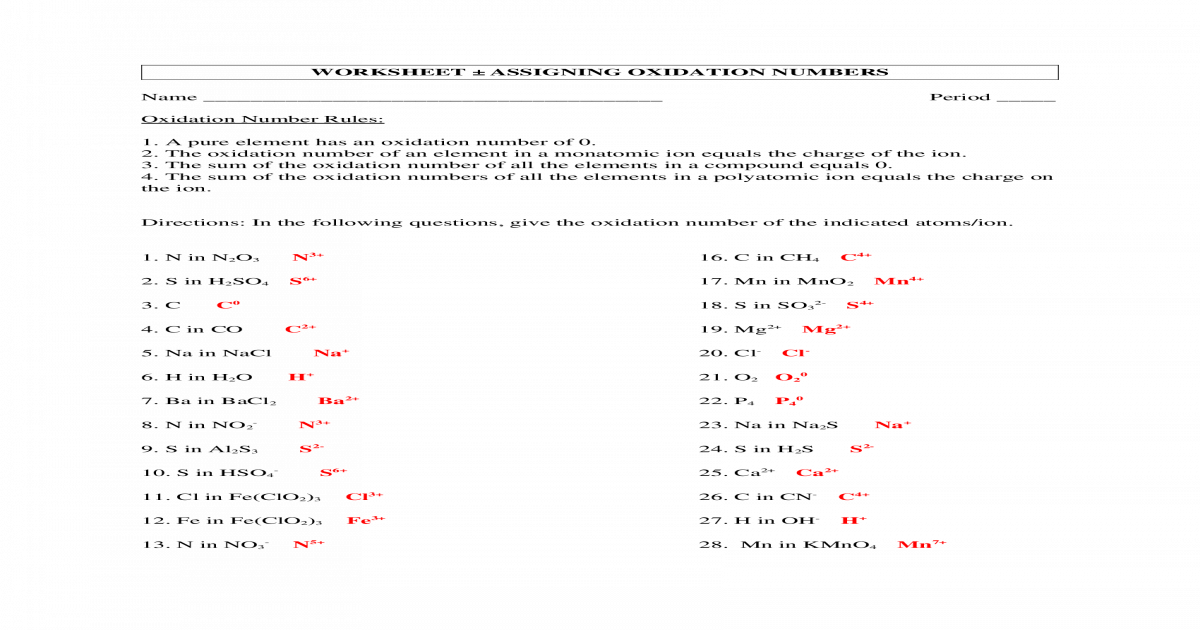
Worksheet Assigning Oxidation Numbers Key
https://img.pdfslide.net/img/1200x630/reader021/image/20170807/577c79841a28abe05492f726.png?t=1627368138
Worksheet Oxidation Numbers Answer Key - A monoatomic ion has an oxidation number equal to its charge For example the oxidation number of the oxygen in the oxide ion O 2 is 2 The sum of the oxidation numbers in a polyatomic ion is equal to the charge on the ion Let s examine the oxidation numbers of some common elements Notice the periodic trend among the main group