What Is The Unit Of Rate Of Zero Order Reaction Feb 7 2019 nbsp 0183 32 Reactions wherein a catalyst is required and is saturated by reactants are generally zero order reactions The unit of the rate constant in a
Jun 30 2023 nbsp 0183 32 What is the rate constant unit of Zero Order Reaction The rate constant of a zero order is given as concentration time or M s where M is the molarity and s is one second The rate constant unit is k mol L 1 s 1 Unit of rate constant for a Zero Order Reaction A unit of zero order reaction is equivalent to a unit of reaction speed The rate constant of the reaction is denoted by k The rate constant of a zero order reaction is given as concentration time
What Is The Unit Of Rate Of Zero Order Reaction
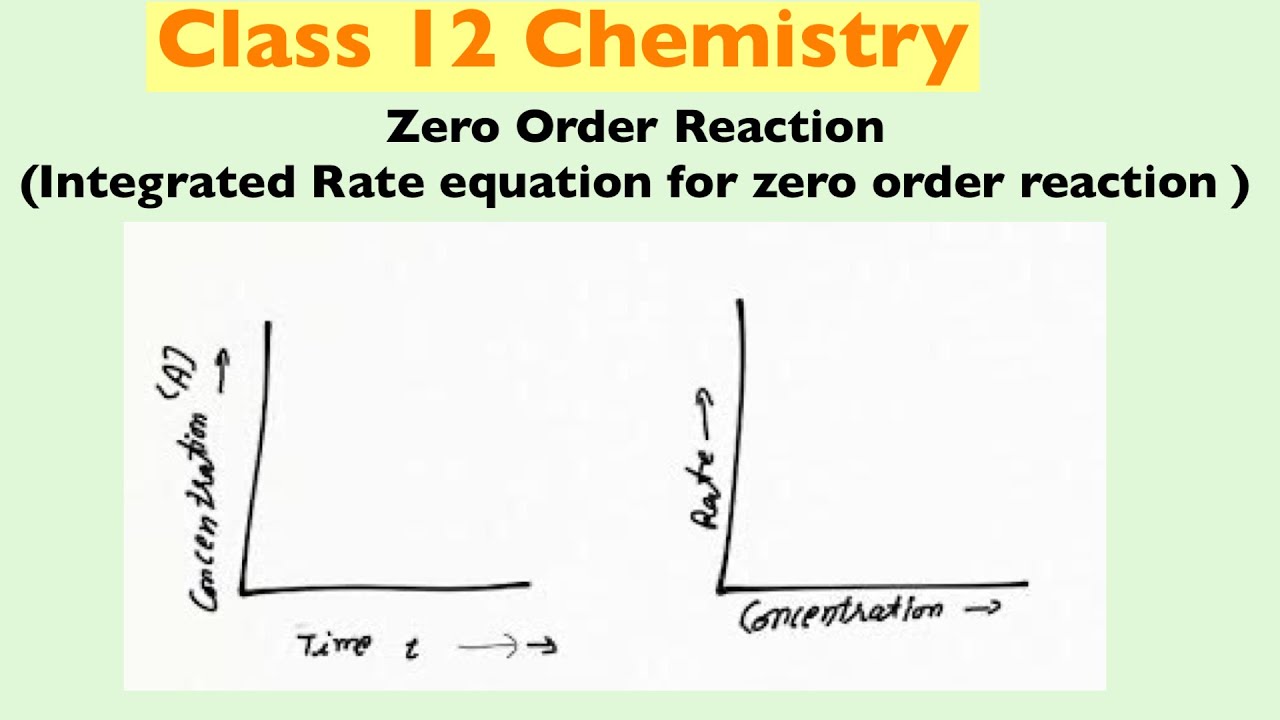
What Is The Unit Of Rate Of Zero Order Reaction
https://i.ytimg.com/vi/KFK4Zwa4ARg/maxresdefault.jpg

How To Determine The Units Of The Rate Constant K Chemical Kinetics
https://i.ytimg.com/vi/1g-vDSWYins/maxresdefault.jpg
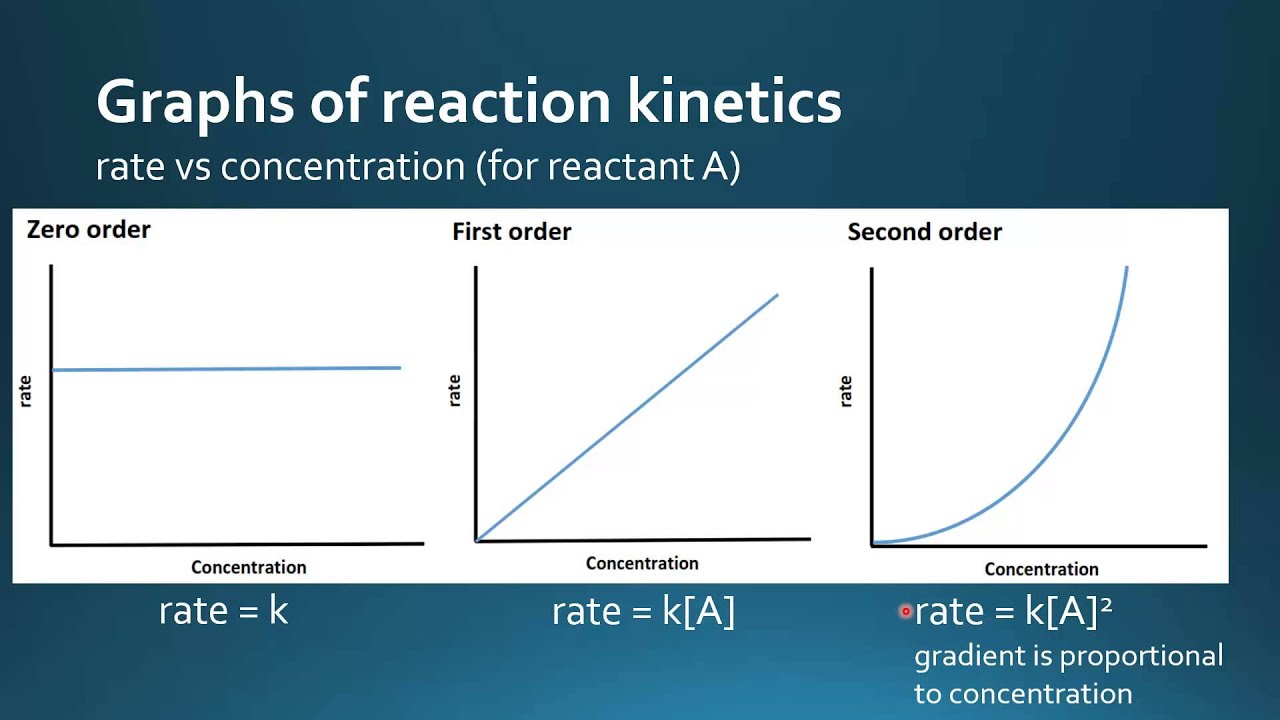
Reactivity 2 2 10 Graphical Representations For Zero First And
https://i.ytimg.com/vi/SuZkqJ79BKk/maxresdefault.jpg
Feb 13 2023 nbsp 0183 32 For zero order reactions the units of the rate constants are always M s In higher order reactions k k will have different units Figure 1 Rate vs For zero order reaction the unit of rate constant is equal to the unit of the rate of the reaction It is M s or mol L s For first order reaction the unit of rate constant is equal to s 1 or min 1
The unit for the reaction rate is moles per liter per second or mol L 1 s 1 Hence the unit for the reaction coefficient is also mol L 1 s 1 The reaction rate is the decrease in reactants concentration per time Therefore the differential form The rate constants for zero first second and nth order reactions are listed below in their units For reaction with order 0 the unit of rate constant is M s 1 or mol L 1 s 1 For reaction with order 1 the units of the rate constant is s 1
More picture related to What Is The Unit Of Rate Of Zero Order Reaction

Integrated Rate Laws Zero First Second Order Reactions Chemical
https://i.ytimg.com/vi/7I0Xg92_eA4/maxresdefault.jpg
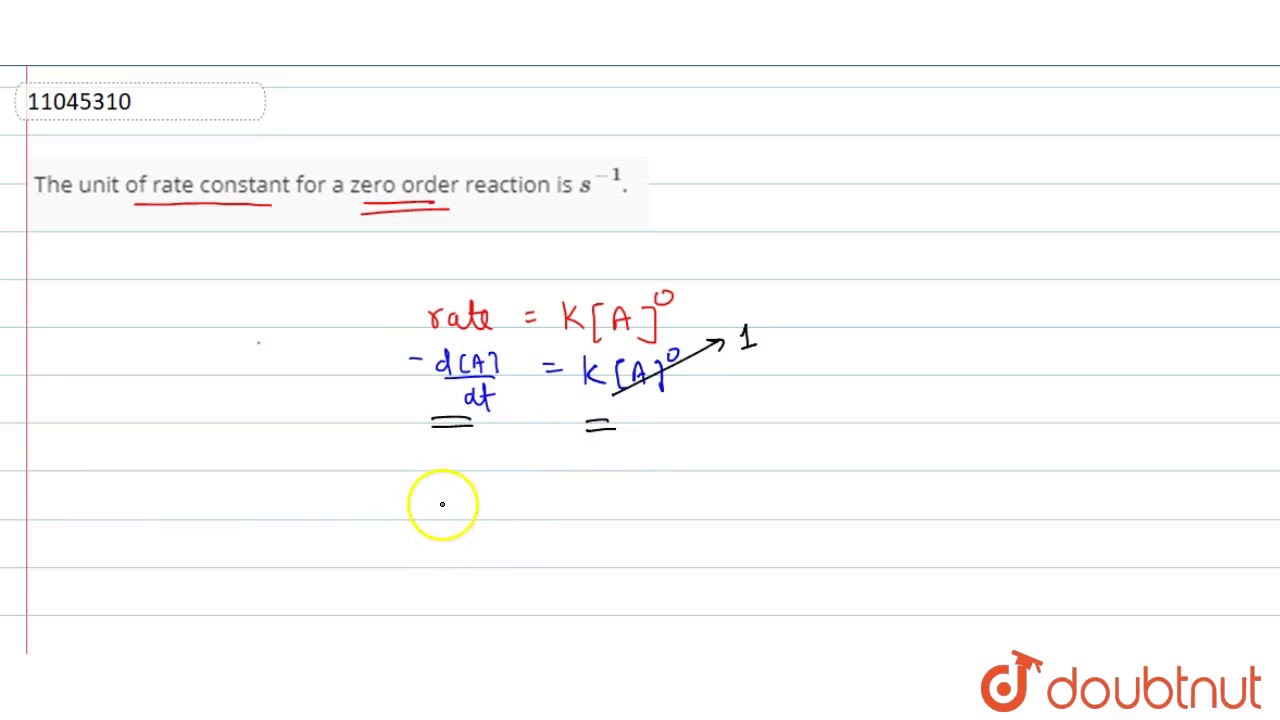
The Unit Of Rate Constant For A Zero Order Reaction Is s 1 YouTube
https://i.ytimg.com/vi/dvmjispfdek/maxresdefault.jpg

Graph Of Integrated Rate Equation Of Zero Order Reaction chemical
https://i.ytimg.com/vi/BZ8Qwg25srA/maxresdefault.jpg
Jul 12 2023 nbsp 0183 32 In a zeroth order reaction the rate constant must have the same units as the reaction rate typically moles per liter per second Although it may seem counterintuitive for the reaction rate to be independent of the reactant Oct 7 2021 nbsp 0183 32 This is the integrated rate law expression for rate constant for zero order reaction For a zero order reaction The rate of reaction is R k A 0 k Hence the velocity constant k has the unit of the rate of the reaction i e mol
Dec 16 2021 nbsp 0183 32 This is an expression of the rate constant of the zero order reaction Using this expression the unit of rate constant for zero order reaction can be determined as k molL 1 sec molL 1 sec 1 Unit of zero order Apr 17 2024 nbsp 0183 32 Basic rate constants are measured in k k depending on the overall orders of the reactions In a zero order reaction the unit of k is M s In a first order reaction the unit of k is

CHEMICAL KINETICS Integrated Rate Equation For Zero Order Reaction
https://i.ytimg.com/vi/q0NWzecNqQU/maxresdefault.jpg
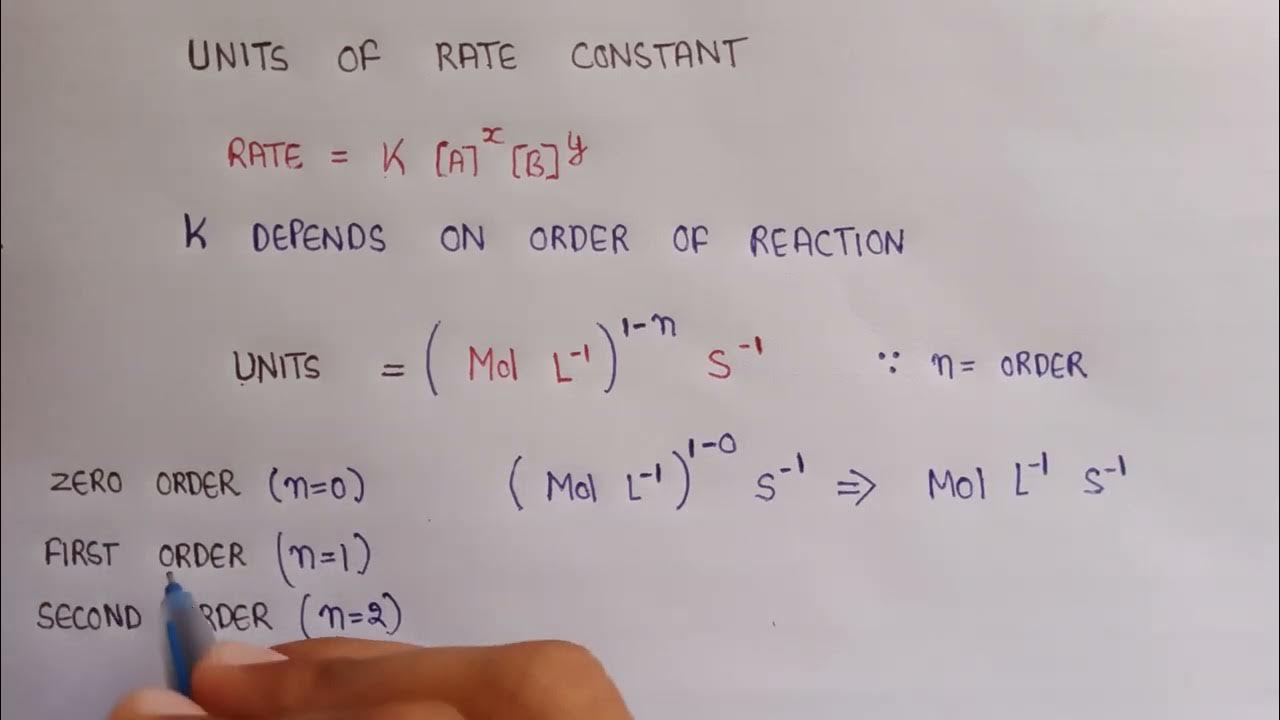
Units Of Rate Constant Calculate Rate Constant Units Units Of First
https://i.ytimg.com/vi/K11dAffAS0U/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLD8eXbkLR_Zj-D2eeR3m4jAqiJcmA
What Is The Unit Of Rate Of Zero Order Reaction - Apr 10 2024 nbsp 0183 32 Rate Constant The rate law for a zero order reaction is rate k where k is the rate constant expressed in concentration time units like M s Half Life The half life of a zero order