Periodic Trends Atomic Radius Worksheet Answers Mar 13 2023 nbsp 0183 32 Know periodic trends of atomic size ionic size ionization energy and electron affinity Understand the reasons for metallic nonmetallic and metalloid character Understand why some acids dissolve in water to make acidic solution while others dissolve in water to make basic solutions
3 What trends do you notice for the atomic radii of Period 3 The atomic radius gets smaller as atomic number increases 4 Explain why this trend occurs Going from left to right across a period the size gets smaller Electrons are in the same energy level but there is more nuclear charge more protons The increased charge pulls electrons 3 Define the following terms atomic radius Describe the periodic trend for atomic radius 4 Define Z eff effective nuclear charge How does this property affect radii of atoms as you move from left to right in a period Does it affect atomic radius as you move down a group
Periodic Trends Atomic Radius Worksheet Answers
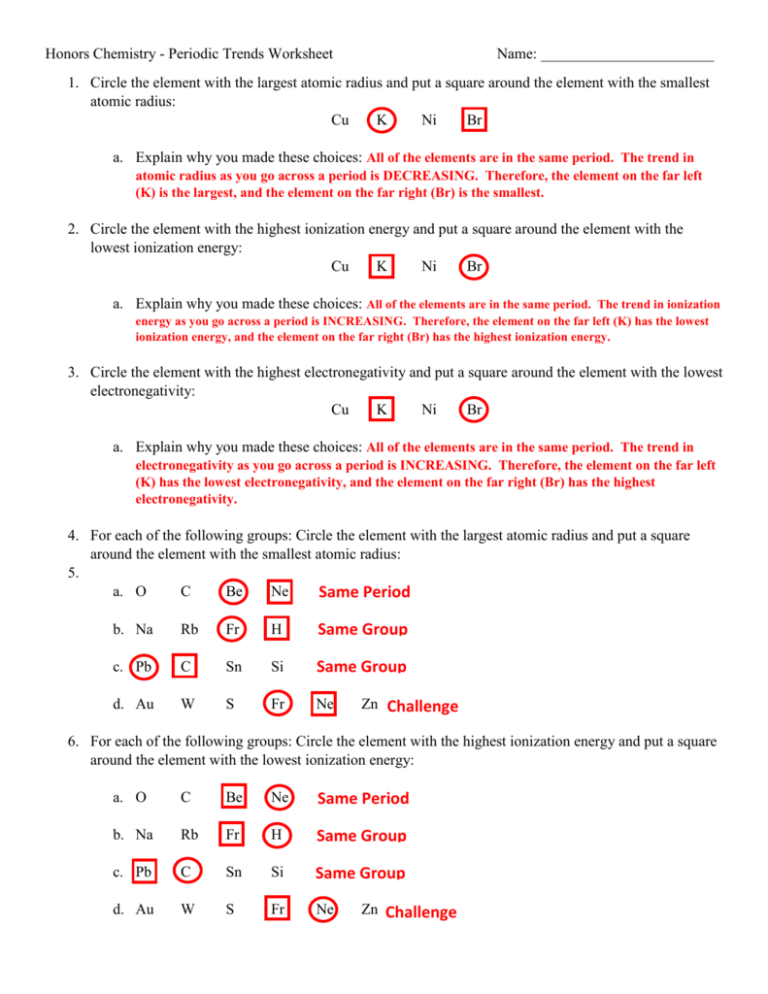
Periodic Trends Atomic Radius Worksheet Answers
https://s3.studylib.net/store/data/006773533_1-4885f6e36ae9fec4ec4b9a1b7b46d97f-768x994.png

50 Periodic Trends Worksheet Answer Key
https://chessmuseum.org/wp-content/uploads/2019/10/periodic-trends-worksheet-answer-key-lovely-20-best-of-periodic-trends-worksheet-answers-key-of-periodic-trends-worksheet-answer-key.png

Periodic Trends Worksheet Atomic Radius Which Atom In Each Pair Has
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/dca0bbd2e76c57fff48a6ed06895d90f/thumb_1200_1553.png
EXTRA PRACTICE Periodic Trends Practice 2 WS ANSWER KEY copy Extra Practice Periodic Trends 1 ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius a Li C b Li Na K 165 L k e Al Cl Ga C I 2 Study with Quizlet and memorize flashcards containing terms like Rank the following elements by increasing atomic radius Carbon Aluminum Oxygen Potassium Rank the following elements by increasing electronegativity Sulfur Oxygen Neon Aluminum Why does fluorine have a higher ionization energy than iodine and more
The graph shows a sudden increase in atomic radius for lithium sodium potassium rubidium and cesium Explain why there is a spike on the graph at element numbers 3 11 19 37 and 55 1 What trend in atomic radius do you see as you go down a group family on the periodic table 2 What causes this trend 3 What trend in atomic radius do you see as you go across a period row on the periodic table 4 What causes this trend 5 Circle the atom in each pair that has the largest atomic radius a Al B b S O c Br Cl
More picture related to Periodic Trends Atomic Radius Worksheet Answers
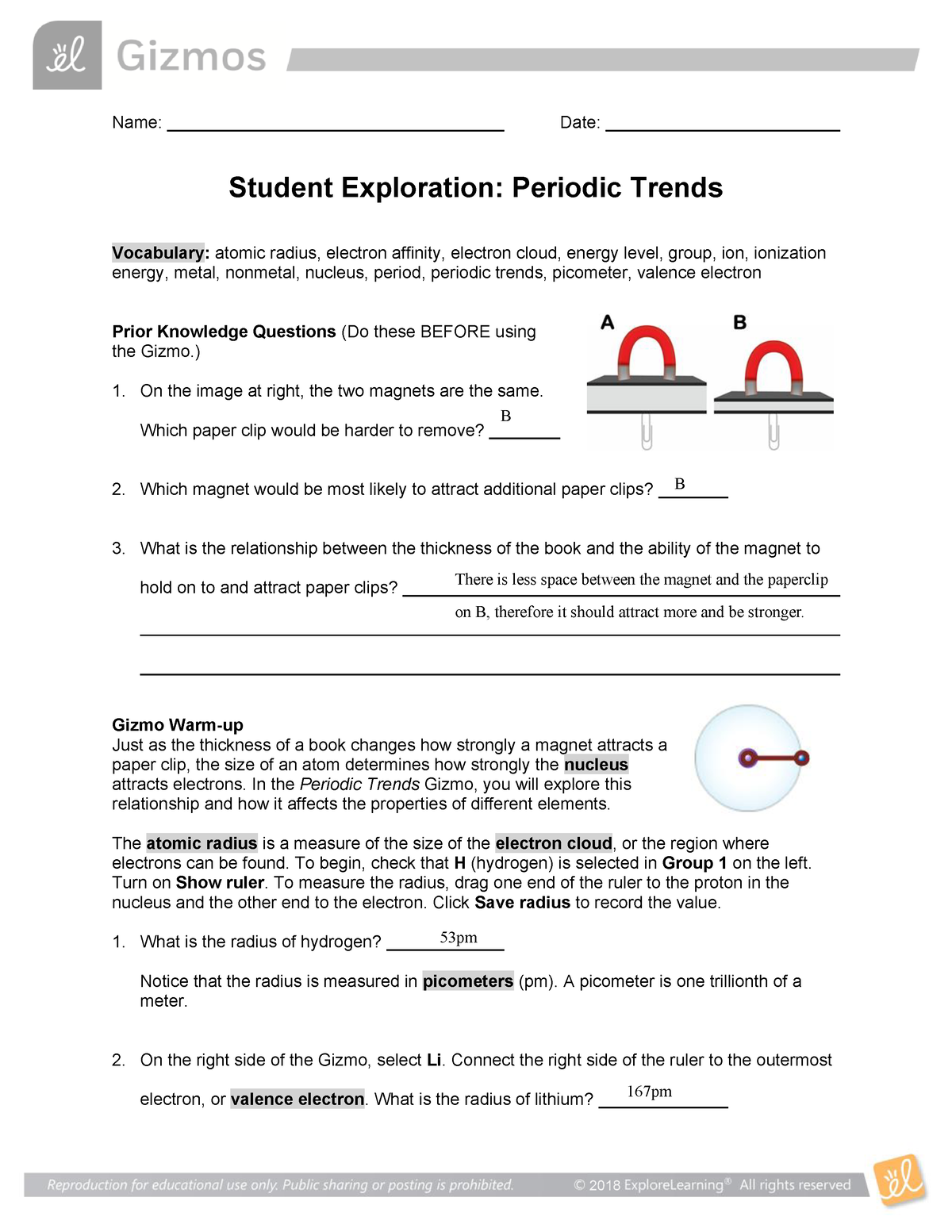
Periodic Trends Gizmo Name Date Student Exploration Periodic
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/45bfcc18b5d618a6ffac477f305a790c/thumb_1200_1553.png

Periodic Trends Notes And Worksheet Set Radius Electronegativity
https://i.pinimg.com/736x/95/66/39/95663994cfff60f789f735eaf2c39570.jpg

Periodic Trends Worksheet 1 Answers 01 160 161 Studocu
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/ab86ad4289c02979b64e49ffd10a8c2d/thumb_1200_1553.png
Periodic Trends I Which of the following in each pair has the larger atomic radius 1 Li or K K 2 Ca or Ni Ca 3 Ga or B Ga 4 O or C C 5 Be or Ba Ba 6 Si or S Si 1 What is the atomic number The number of protons found in an element 2 What is the atomic radius How do you think chemists measure it The radius of an atom or its size Normally represented as covalent radii chemists determine this by measuring the distance between one element and another when bonded 3
This online quiz is intended to give you extra practice in identifying different periodic trends such as atomic radius ionization energy and electron affinity This quiz aligns with the following NGSS standard s HS PS1 1 HS PS1 2 Select your preferences below and click Start to give it a try Jan 28 2019 nbsp 0183 32 The tiered levels of questions and reflection may be used to differentiate between introductory advanced first year and AP chemistry Students create their own diagrams using blank periodic tables of the main group elements in the first four periods Four models are developed atomic radius ionic radius ionization energy and electronegativity
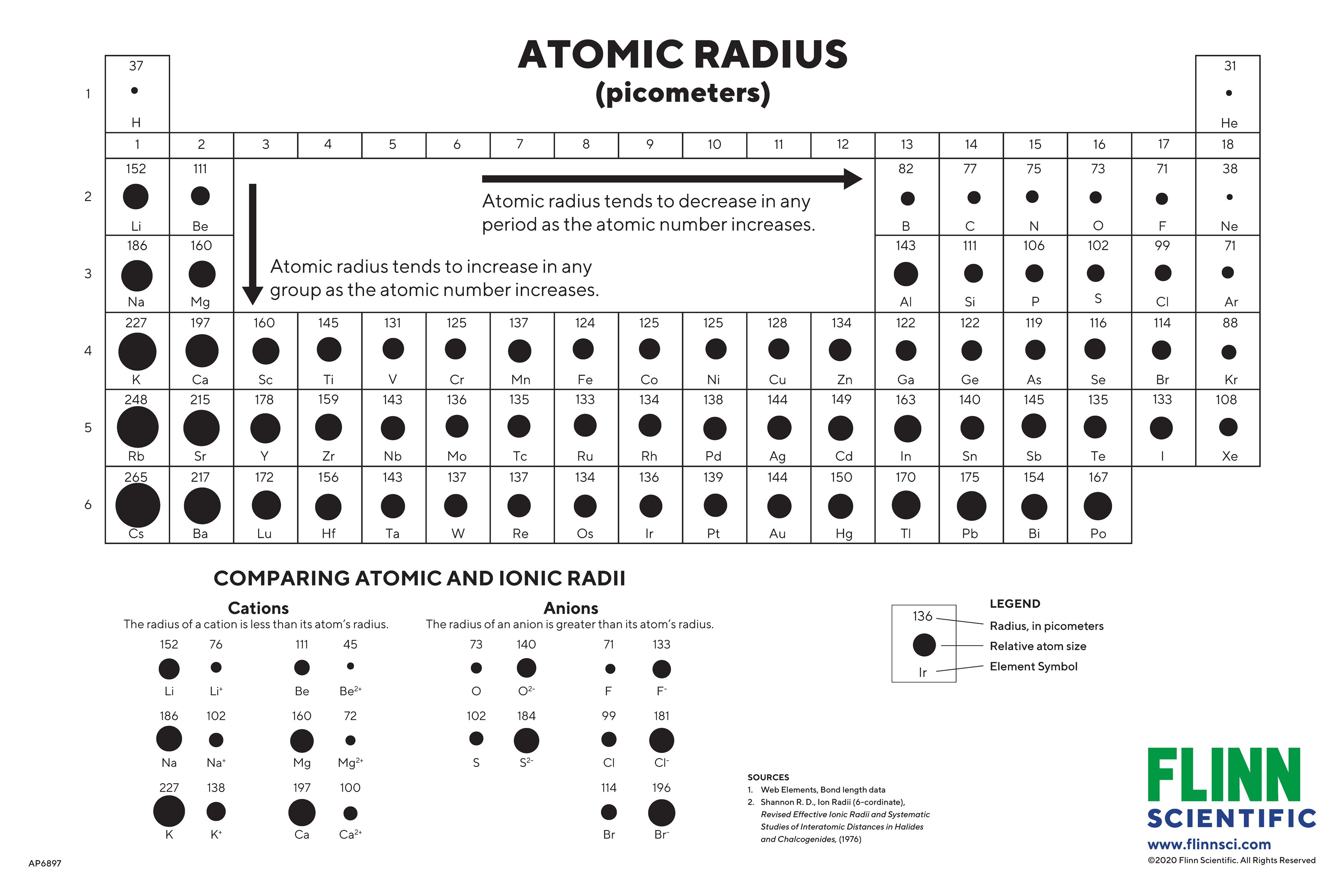
Atomic Radius Periodic Table Trend Elcho Table
https://www.flinnsci.com/globalassets/flinn-scientific/all-product-images-rgb-jpegs/ap6897_atomicsizesandradiichart_48x32.jpg
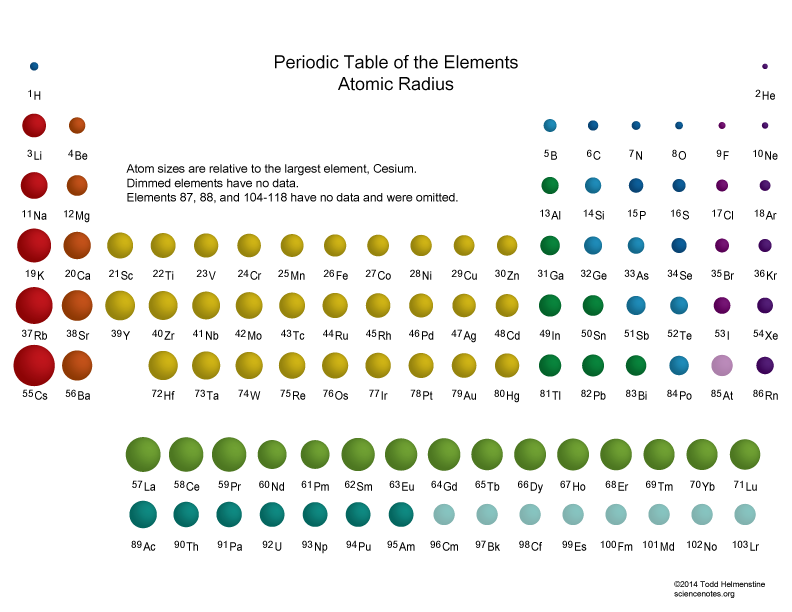
Periodic Trends Atomic Radius Worksheet Answers
http://periodictrendsforhonorschemistry.weebly.com/uploads/4/6/0/8/46086741/7496628_orig.png
Periodic Trends Atomic Radius Worksheet Answers - Jun 11 2021 nbsp 0183 32 Predict the variation in atomic radius in the periodic table Describe specific reasons for this variation across a period and within a group Predict differences in atomic radius and ionic radius for the same element predict differences in ionic radius for various oxidation states of the same element