What Is The Relationship Between Temperature And The Rate Of Chemical Reactions Increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions It is only these collisions possessing at least the activation energy for the reaction
Most of the chemical reactions show a change in their reaction rate with varying temperature It has been observed that the rate constant for a chemical Jan 10 2021 nbsp 0183 32 Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates The Arrhenius equation describes the relation between a reaction s rate constant and its activation
What Is The Relationship Between Temperature And The Rate Of Chemical Reactions

What Is The Relationship Between Temperature And The Rate Of Chemical Reactions
https://i.ytimg.com/vi/U3HVaBgj6gw/maxresdefault.jpg

16 2 Effect Of Temperature On The Rate Constant K HL YouTube
https://i.ytimg.com/vi/cJ9Z3h_6ne8/maxresdefault.jpg
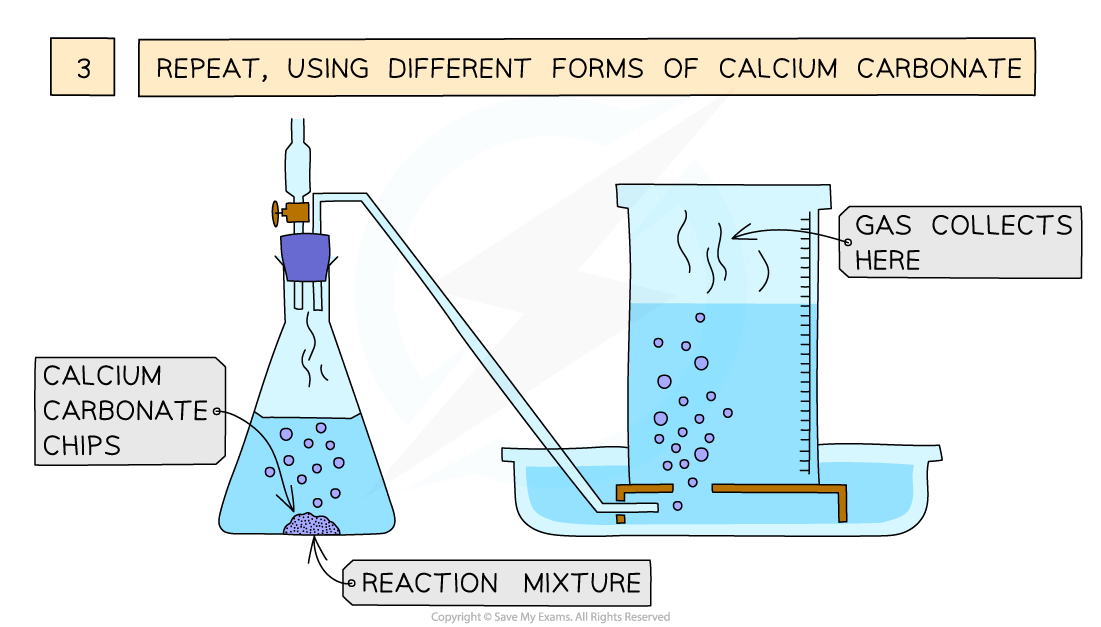
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 3 2 4 Practical Effect Of
https://oss.linstitute.net/wechatimg/2022/11/3.2.1-Effect-of-Surface-Area-on-a-Reaction-Rate-3.png
Oct 29 2020 nbsp 0183 32 Temperature is often the factor that has the greatest effect on reaction rate Increasing temperature gives particles kinetic energy so they bounce around more quickly and are more likely to combine Use the postulates of collision theory to explain the effects of physical state temperature and concentration on reaction rates Define the concepts of activation energy and transition state Use the Arrhenius equation in
Jul 9 2023 nbsp 0183 32 This equation shows the dependence of the rate of a chemical reaction on the temperature The Arrhenius factor also known as the frequency factor or pre exponential factor is represented by A E a is the activation What effect does temperature have on reaction rates With a little sodium thiosulfate and hydrochloric acid students will be able to discover just that Complete the table provided to give a clear view of the data collected and
More picture related to What Is The Relationship Between Temperature And The Rate Of Chemical Reactions
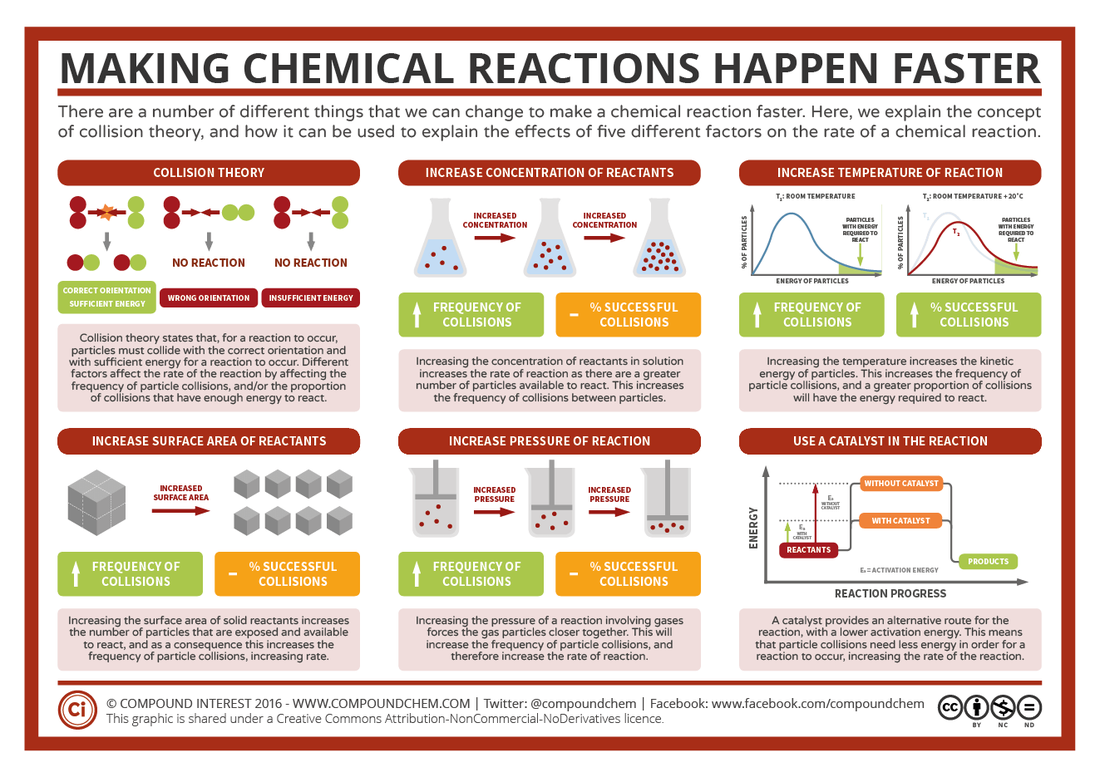
Kinetic Chemistry Connect With Chemistry World
http://chemistrysabras.weebly.com/uploads/6/0/4/8/60482409/6590098_orig.png
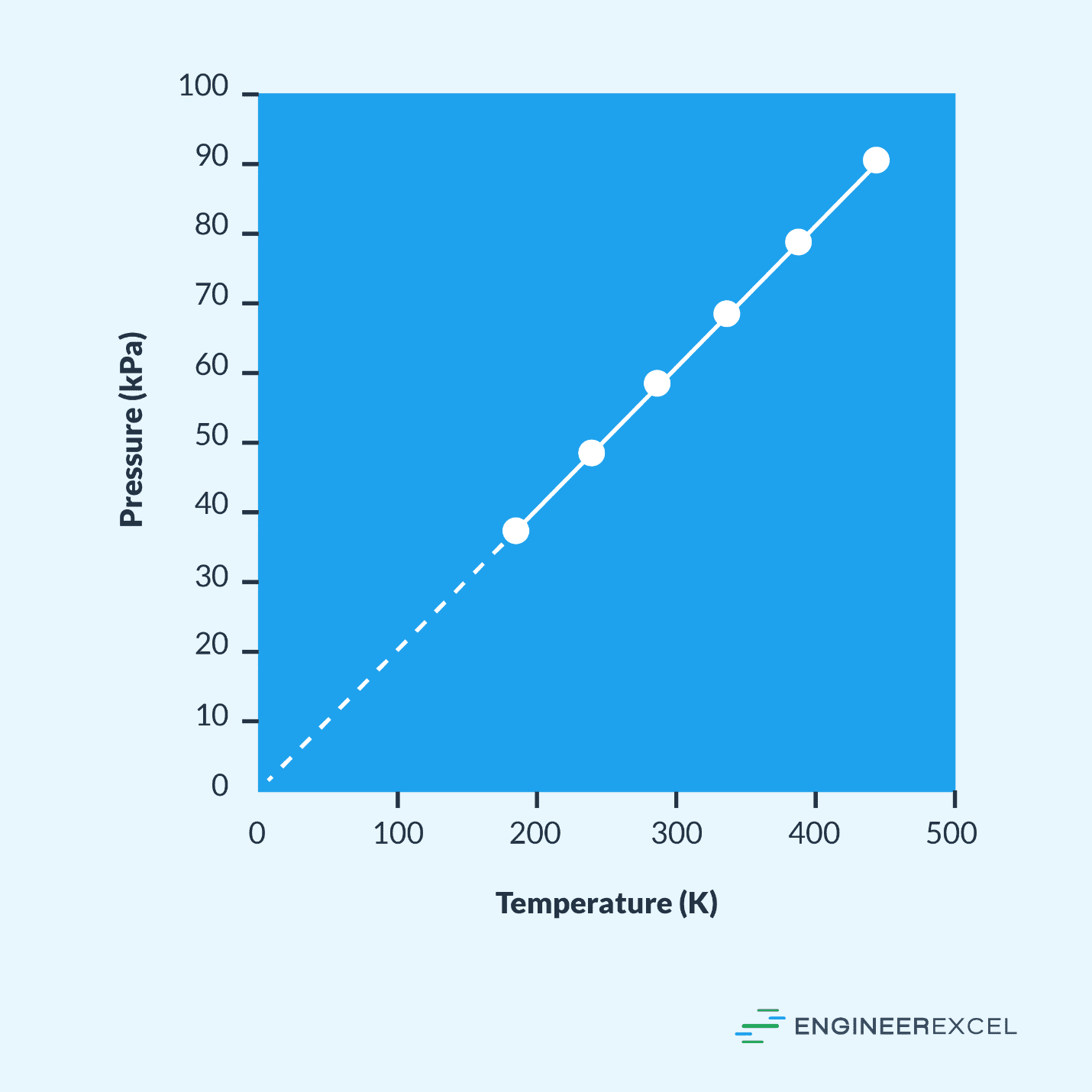
Pressure Temperature Graphs Explained EngineerExcel
https://engineerexcel.com/wp-content/uploads/2023/03/relationship-between-the-pressure-and-temperature.webp
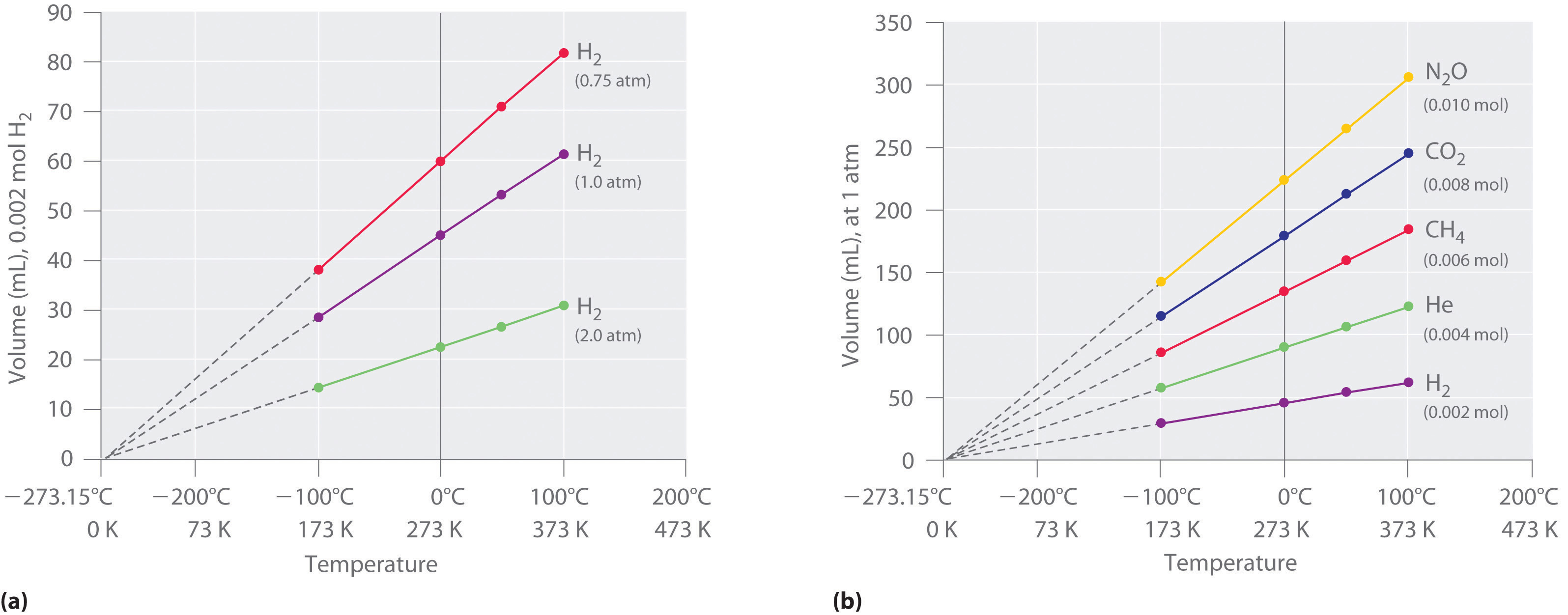
The Relationship Between Temperature And Volume Charles Law
https://chem.libretexts.org/@api/deki/files/30568/eed54a71ea87565548dd591aff65c436.jpg?revision=3
Mar 8 2020 nbsp 0183 32 How does temperature affect the rate of reaction The effect of temperature on rate of reaction is to speed it up mainly by increasing the number of particles that achieve enough energy to cross the activation energy For example the reaction rates of many reactions that occur at room temperature approximately double with a temperature increase of only 10 176 C In this section we will use the collision model to analyze this relationship between temperature
The effect of temperature on the rates of chemical reactions This page explains why changing the temperature changes reaction rates and introduces the concept of activation energy The Mar 15 2023 nbsp 0183 32 Temperature directly affects the rate of a chemical reaction Raising the temperature causes the molecules to move faster and collide more often which in turn

8 The Graph Shows The Relationship Between Temperature In Degrees
https://us-static.z-dn.net/files/d51/eea546bd6615f19e00016263e390acbf.png

Under Marcos Philippines Human Rights and US Relations Improve WPR
https://www.worldpoliticsreview.com/wp-content/uploads/2022/11/marcos-philippines-human-rights-us-relations-11222022-1.jpg
What Is The Relationship Between Temperature And The Rate Of Chemical Reactions - Apr 27 2024 nbsp 0183 32 We can represent the effect of temperature on reaction rate using a rate of reaction graph The graph line for the higher temperature shows a higher rate of reaction than the line