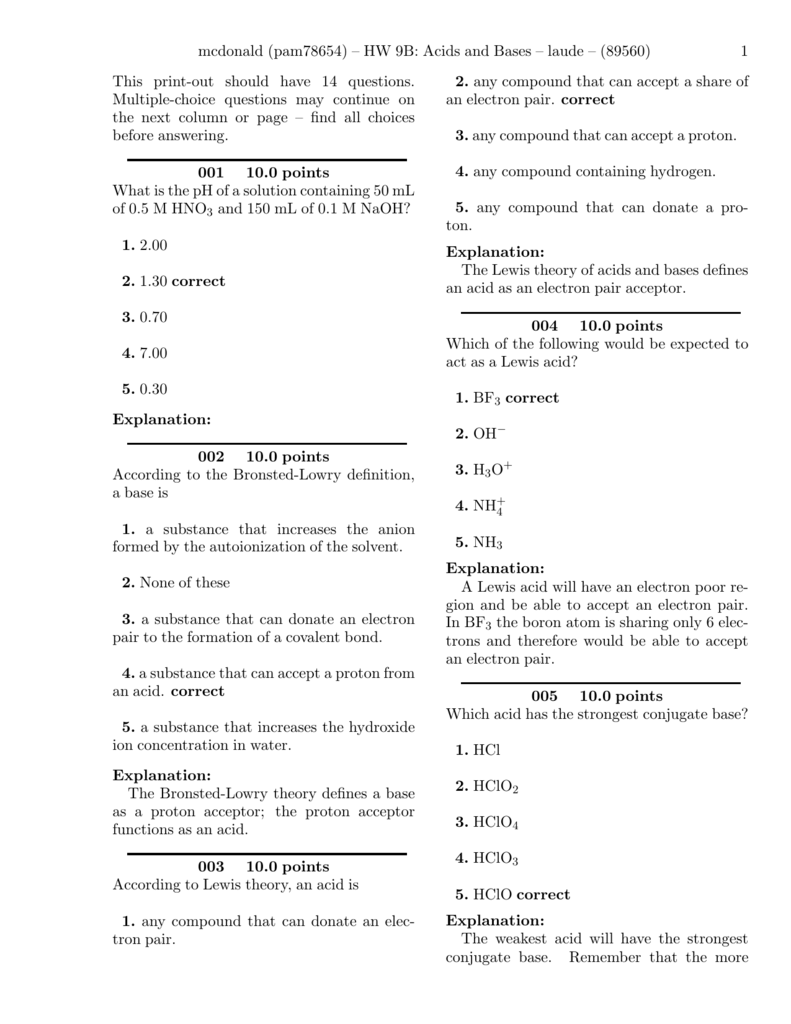
Conjugate Acids And Bases Worksheet Conjugate Pairs Practice Questions Identify the acid base conjugate acid and conjugate base for each of the following Complete the equation for the reaction of each of the following with water Then Indicate whether each reaction is explained by Arrhenius Br 248 nsted Lowry or both
Phosphate Ion In the following equations labelthe correct Br 248 nsted Lowery acid base Then label the conjugate acid base The donating acid and the base it becomes are called conjugate acid base pairs The base on the left accepts a proton H and becomes an acid on the right
Conjugate Acids And Bases Worksheet

Conjugate Acids And Bases Worksheet
https://i.pinimg.com/originals/79/ed/65/79ed65fd4c144746604673944b9151ba.jpg

18 Acids And Bases
https://s3.studylib.net/store/data/008924205_1-35c5d9d9251845ea9d6591c797d5ce44-768x994.png
Worksheet Conjugate Acid Base Pairs Worksheet Grass Fedjp Worksheet
http://s3.studylib.net/store/data/008360426_1-ac41bae5b056c3b552ea349a12c3dd74.png
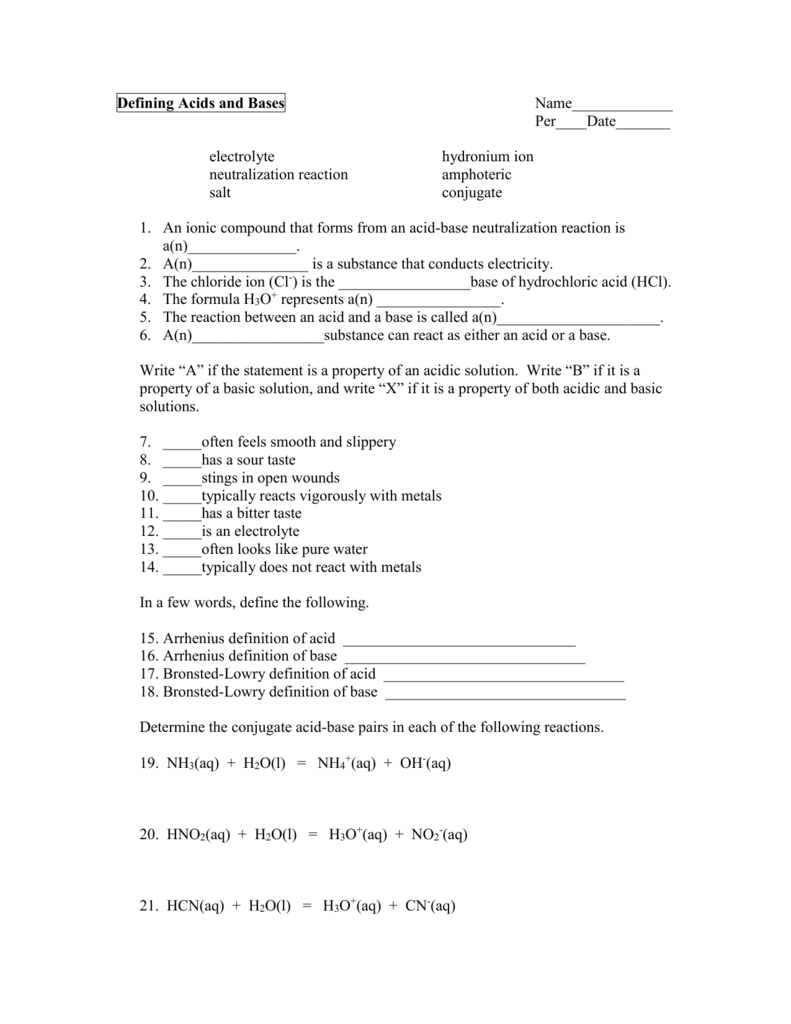
An acid is defined as a proton H donor while a base is a proton acceptor The substance that is produced after an acid has donated its proton is called the conjugate base while the substance formed when a base accepts a proton is called the conjugate acid 4 Worksheet Acids and bases 2 1 Write a balanced equation for the reaction between nitric acid and water and indicate the conjugated acid base pairs 2 4 25 cm3 water is added tot 75 cm3 of a 0 13 mol 183 dm 3 sulphuric acid solution Calculate the concentration of the diluted solution
The substance that is produced after an acid has donated its Bases accept protons proton is called the conjugate while the substance formed when a base accepts a proton is called the conjugate acid Conjugate Acids and Bases Practice Label the acid A base B conjugate acid CA and conjugate base CB in each of the following reactions HC2H3O2 aq H2O l NH4 aq OH aq
More picture related to Conjugate Acids And Bases Worksheet

Naming Acids And Bases Worksheet Answers
https://i2.wp.com/s3.studylib.net/store/data/007859161_2-ad0ad00dc184e68385d4ee19a57c83ad.png

Acid And Base Worksheet Answers
https://s3.studylib.net/store/data/025300249_1-189b539b450321cd390179810311a584.png

16 1 Conjugate Acids And Bases Worksheet YouTube
https://i.ytimg.com/vi/avPXDhluQ3o/maxresdefault.jpg
Worksheet 4 2 Conjugate Acid Base Pairs Complete each reaction Label each reactant or product as an acid or base 1 HCN H 2 O H 3 O CN 2 HCl H 2 O H 3 O Cl 3 HF H 2 O H 3 O F 4 F H 2 Acids amp Bases Worksheet Objectives Identify acids amp bases and their conjugates and identify them as strong or weak Calculate pH ionization constants and titration problems Write acid base reaction equations amp identify the substances participating Predict the properties of
II Conjugate Acids and Bases In each reaction identify the acid base conjugate acid and conjugate base Then write which acid base theory or theories describe the reaction a NH3 H2O NH4 OH base acid conj Acid conj base Theory Bronsted b NH4 H 2O NH3 H3O acid base conj base conj acid Theory Bronsted c 2NaOH Conceptual Questions Acids Bases and Conjugates Miscellaneous 1 In the Br 248 nsted Lowry definition of acids and bases an acid a is a proton donor b is a proton acceptor c forms stable hydrogen bonds d breaks stable hydrogen bonds e corrodes metals 2 In the Br 248 nsted Lowry definition of acids and bases a base

Acids And Bases Worksheets Pdf
https://www.housview.com/wp-content/uploads/2019/07/conjugate_acids_and_bases_worksheet_conjugate_acid_base_pairs_worksheet_conjugate_acid_base_pairs_worksheet_conjugate_acid_base_pairs_chem_worksheet_9.jpg
Worksheet Conjugate Acid Base Pairs Worksheet Grass Fedjp Worksheet
http://s3.studylib.net/store/data/007418728_1-1cfabe661f368e076196c52c77074807.png
Conjugate Acids And Bases Worksheet - Acids Bases amp pH Worksheet PROPERTIES OF ACIDS AND BASES 1 Acids taste and bases taste bases also feel 2 Acids turn litmus paper bases turn litmus paper and phenolphthalein 3 Circle Weak acids are strong weak electrolytes