What Does Not Affect Rate Of Chemical Reaction Volume itself doesn t directly affect the rate of reaction changes in volume can affect pressure and in cases involving gaseous reactants the equilibrium position which can indirectly influence the rate of reaction
May 25 2021 nbsp 0183 32 Chemists have identified many factors that affect the rate of a reaction The rate or speed at which a reaction occurs depends on the frequency of successful collisions Remember a successful collision occurs when two reactants collide with enough energy and with the right orientation Effect of concentration on reaction rate According to the collision theory the rate of reaction increases with the increase in the concentration of the reactants As per the law of mass action the chemical reaction rate is directly proportional to the concentration of reactants
What Does Not Affect Rate Of Chemical Reaction

What Does Not Affect Rate Of Chemical Reaction
https://i.ytimg.com/vi/qIvPSuUsgJU/maxresdefault.jpg

How Does Surface Area Affect Rate Of Reaction YouTube
https://i.ytimg.com/vi/lukSSS9Hfaw/maxresdefault.jpg
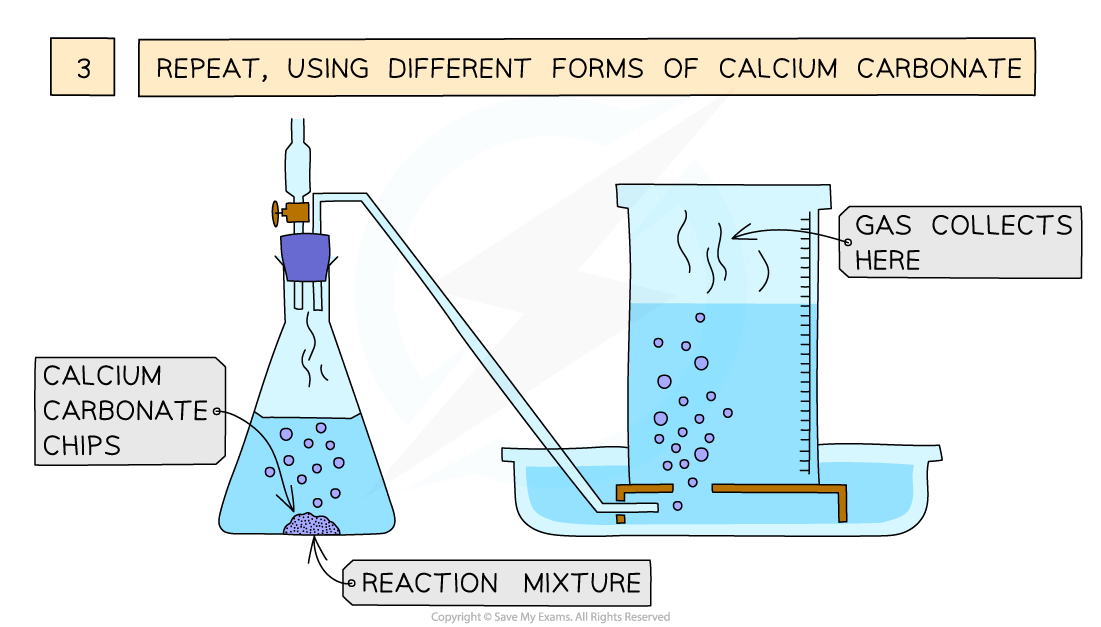
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 3 2 4 Practical Effect Of
https://oss.linstitute.net/wechatimg/2022/11/3.2.1-Effect-of-Surface-Area-on-a-Reaction-Rate-3.png
Sep 18 2020 nbsp 0183 32 The molecularity of reactants does not affect the rate of the reaction Molecularity touch on the number of material of the reactants that must collide to accompany a reaction Change in total heat is the gain or loss of energy that come about in Sep 12 2022 nbsp 0183 32 The rate of a chemical reaction is affected by several parameters Reactions involving two phases proceed more rapidly when there is greater surface area contact If temperature or reactant concentration is increased the rate of a given reaction generally increases as well
There are many factors that influence the reaction rates of chemical reactions include the concentration of reactants temperature the physical state of reactants and their dispersion the solvent and the presence of a catalyst The rate of reaction can be found by measuring the amount of product close product A chemical which is made in a chemical reaction Products are written on the right of a chemical equation after
More picture related to What Does Not Affect Rate Of Chemical Reaction

Catalyst
https://sciencenotes.org/wp-content/uploads/2022/09/Catalysts-and-Catalysis.png
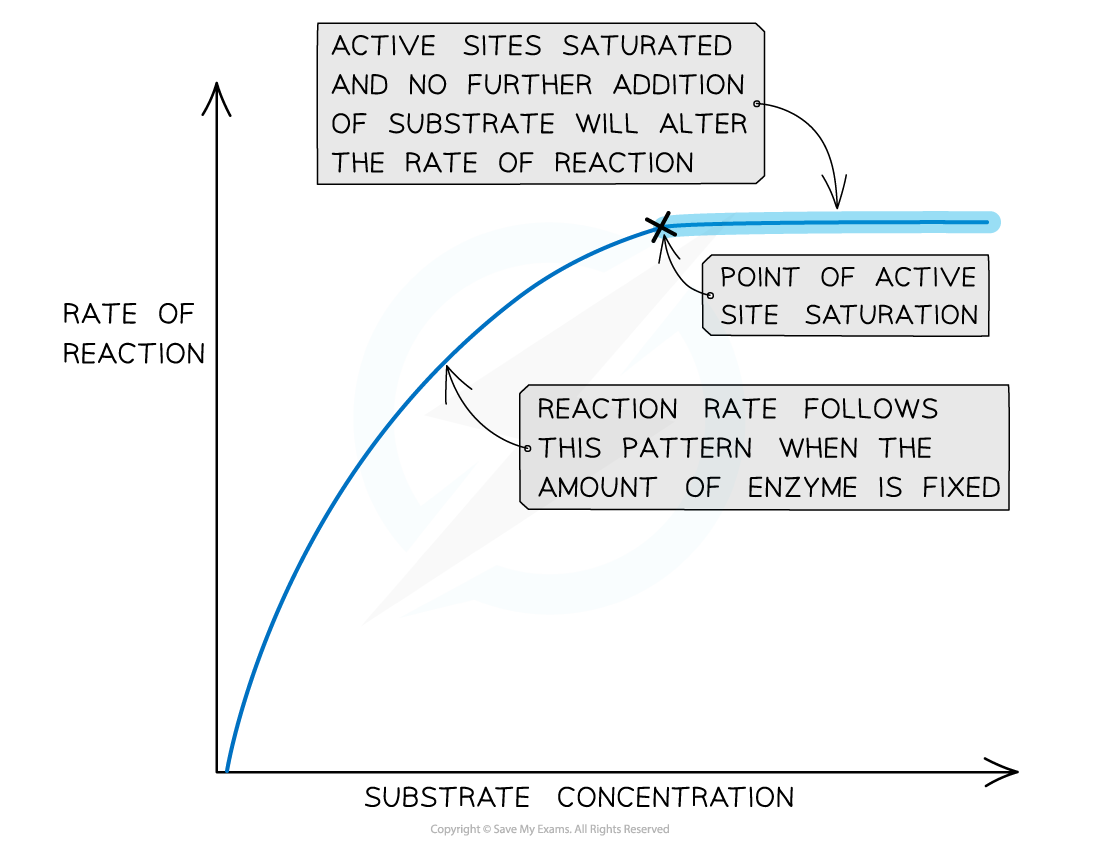
CIE A Level Biology 3 2 4 Rate Substrate Concentration
https://oss.linstitute.net/wechatimg/2022/08/The-effect-of-substrate-concentration-on-an-enzyme-catalysed-reaction-3.png

Concentration Enzyme Reaction Rates Effects Role Expii
https://d20khd7ddkh5ls.cloudfront.net/enzyme_activity_concentration.jpg
There are four factors that affect the rate speed of a chemical reaction catalyst Catalysts are substances that speed up chemical reactions but can be recovered chemically unchanged at the Which of the following factors does not affect the rate of a chemical reaction A Catalysts B surface area C temperature D concentration or E color of the reactants A chemical reaction can happen when particles collide with each other
The rate of a chemical reaction is proportional to concentration of reactants present As reactants are used up during the process the rate will decrease and the reaction slows down Sep 24 2024 nbsp 0183 32 Use our revision notes to understand factors affecting the rate of reaction including temperature concentration surface area and catalysts Learn more Increasing the concentration of a solution or gas pressure increases the rate of reaction Increasing the surface area increases the rate of reaction
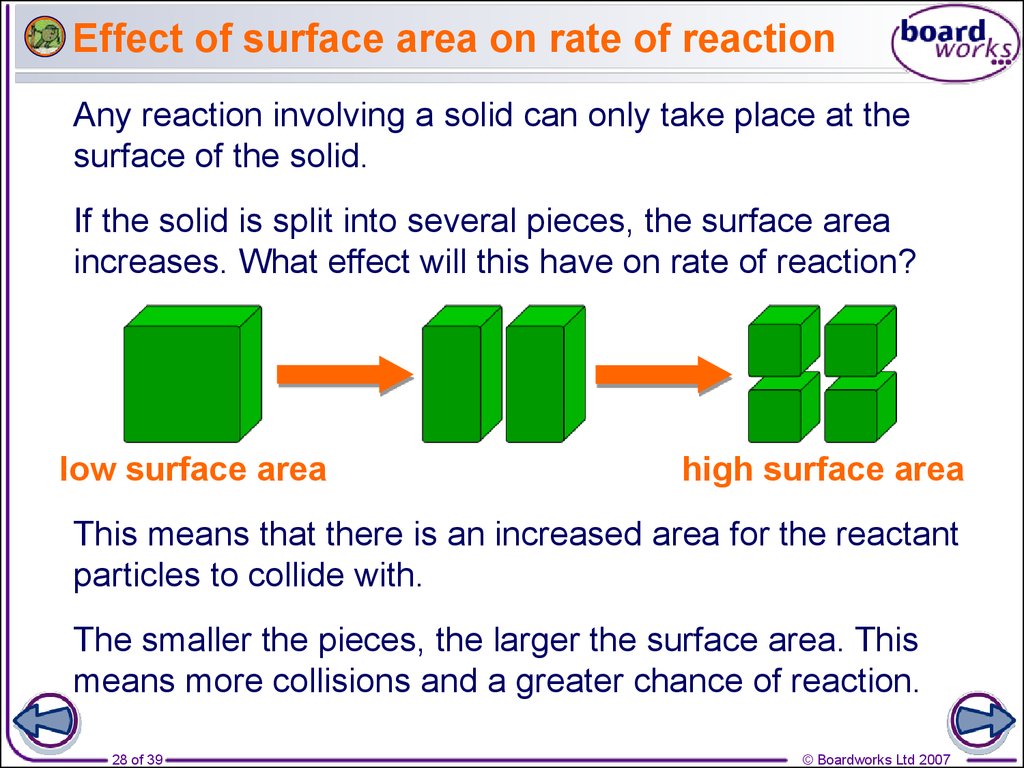
Rates Of Reaction Online Presentation
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-27.jpg
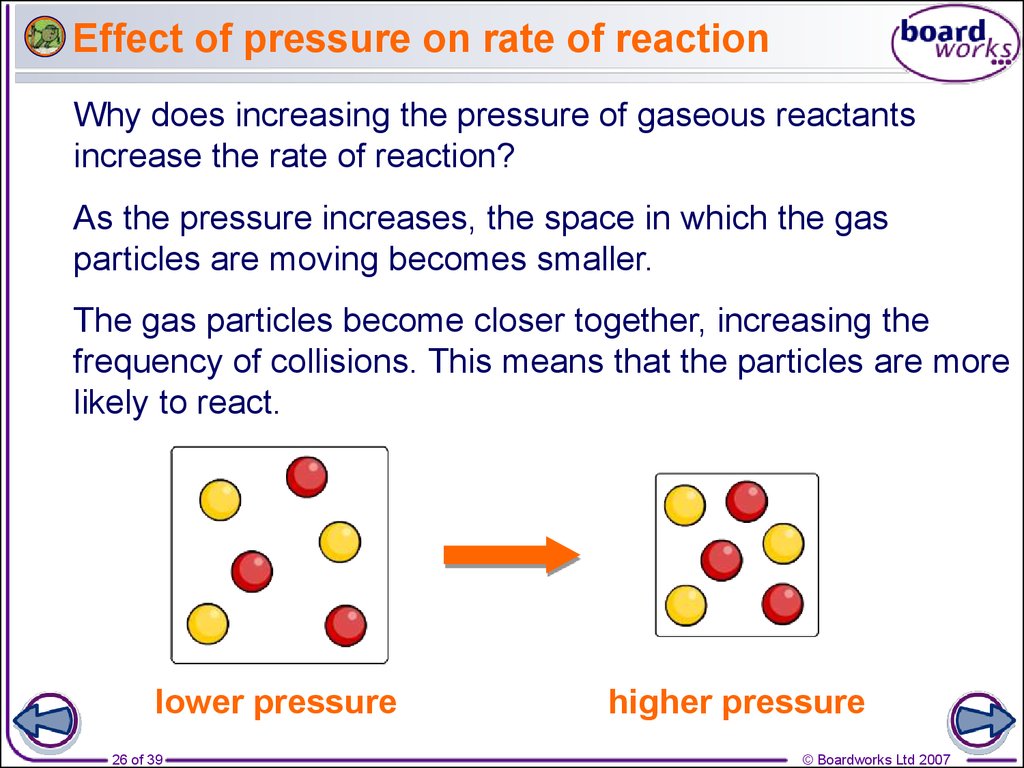
Rates Of Reaction Online Presentation
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-25.jpg
What Does Not Affect Rate Of Chemical Reaction - Feb 16 2020 nbsp 0183 32 Chemical reactions take place when particles collide with enough energy and in the correct orientation Without collisions no reaction is possible The rate of reaction is affected by reactant concentration temperature pressure