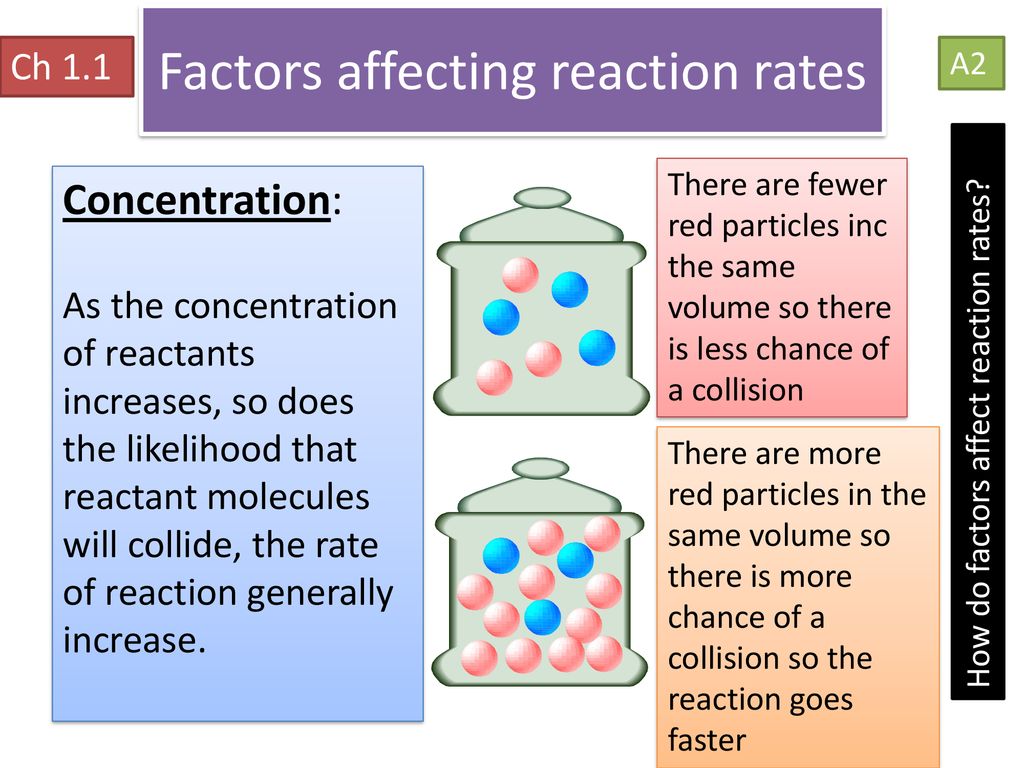
Which Of The Following Does Not Affect The Rate Of Chemical Reaction Amount of product does not affect reaction rate as the concentration of product do not appear in the rate law expression On the other hand Concentration Pressure and Temperature affects
Molecularity of the reaction does not influence the rate of reaction It is related to the order of the reaction The temperature of the reaction concentration or partial pressures of the reactants The concentration of reactants affects the rate of a chemical reaction except in zero order reactions where the rate is independent of the concentration The temperature affects the rate
Which Of The Following Does Not Affect The Rate Of Chemical Reaction
Which Of The Following Does Not Affect The Rate Of Chemical Reaction
https://oss.linstitute.net/wechatimg/2022/11/3.2.1-Effect-of-Surface-Area-on-a-Reaction-Rate-3.png

Catalyst
https://sciencenotes.org/wp-content/uploads/2022/09/Catalysts-and-Catalysis.png

Concentration Enzyme Reaction Rates Effects Role Expii
https://d20khd7ddkh5ls.cloudfront.net/enzyme_activity_concentration.jpg
Which of the following does not affect the rate of reaction Select one O a concentration of products O b concentration of reactants O c temperature O d nature of reacting species O e catalyst A reaction is 2nd order with respect to There are 2 steps to solve this one The rate of a chemical reaction is influenced by several factors Analyze each of the options given a Not the question you re looking for Post any
Apr 7 2019 nbsp 0183 32 The reaction CH3 3COOC CH3 3 g 2CH3COOCH3 g C2H6 g is carried out at constant volume with P0 Which of the following factors does not affect the rate of a chemical reaction A Catalysts B surface area C temperature D concentration or E color of the reactants A chemical reaction can happen when particles collide with each
More picture related to Which Of The Following Does Not Affect The Rate Of Chemical Reaction
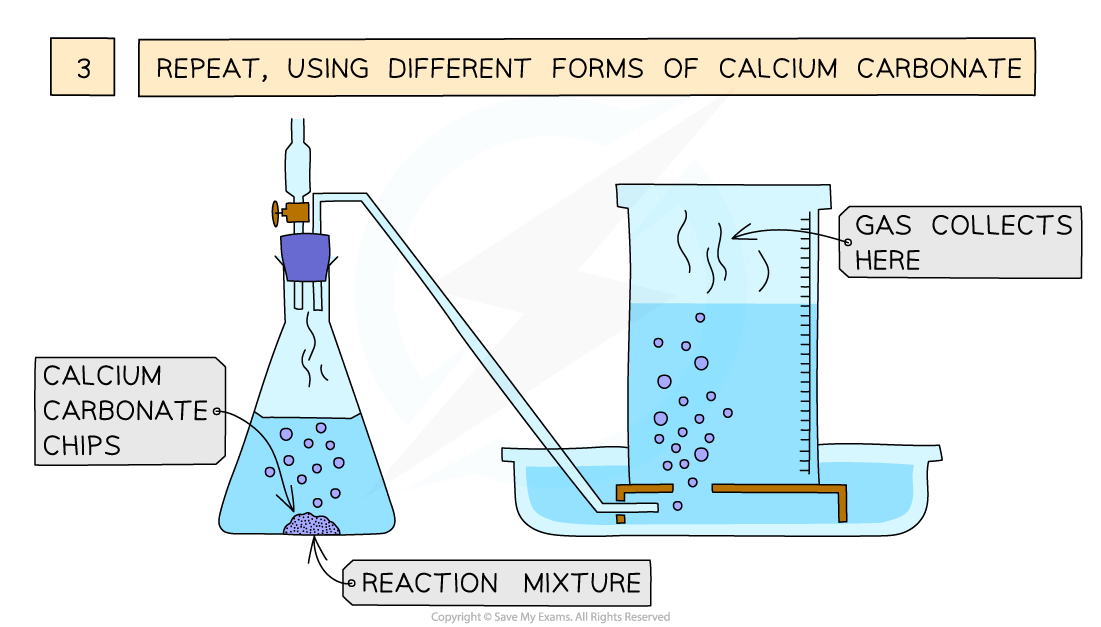
Rates Of Reaction
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-22.jpg
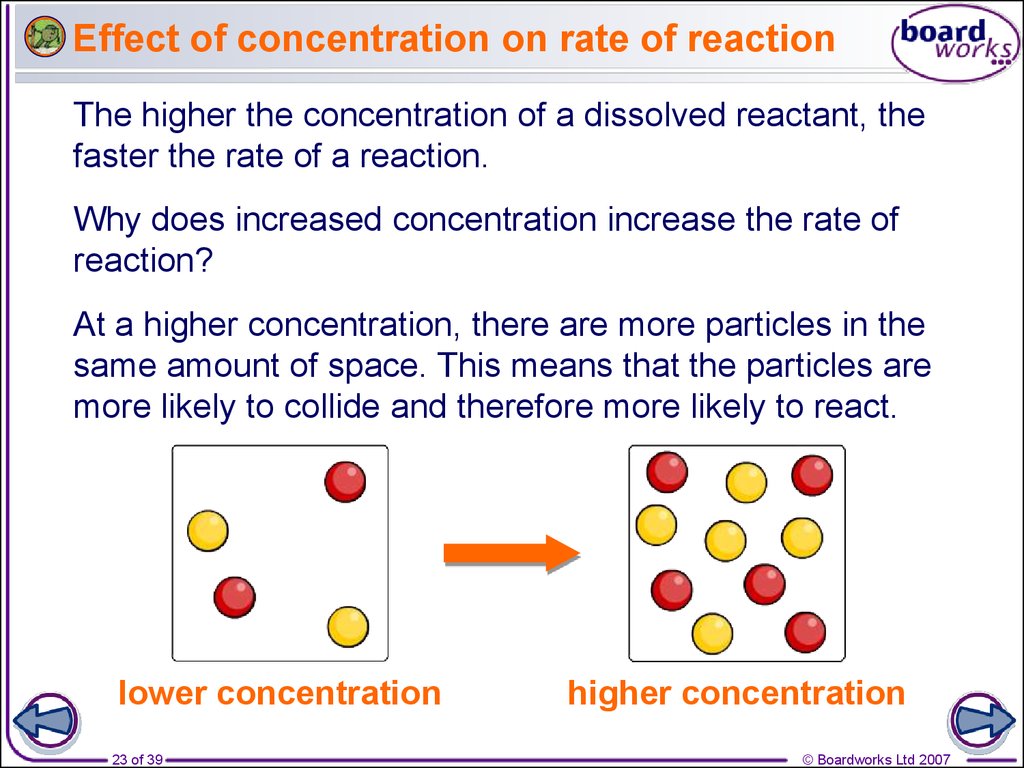
Rates Of Reaction Online Presentation
https://cf.ppt-online.org/files/slide/q/q3gKkwJHLrQzvFxOdViAPehSbMlUsTZYWno2C9/slide-27.jpg
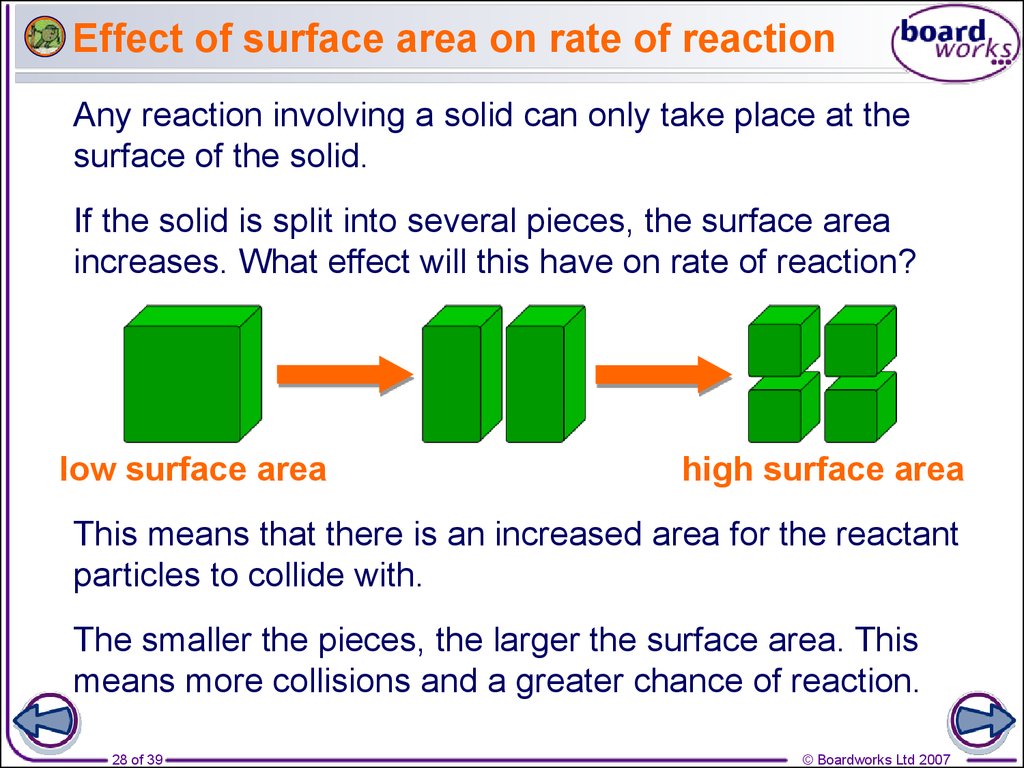
Chapter 1 Rate Of Reaction Ppt Download
https://slideplayer.com/slide/15098838/91/images/6/Factors+affecting+reaction+rates.jpg
Aug 24 2023 nbsp 0183 32 The factor that DOES NOT affect the rate of a chemical reaction among the given options is the size of the equilibrium constant All other factors directly influence the rate of the Jul 1 2023 nbsp 0183 32 While the H of reaction is important in determining the overall energy changes in a reaction it does not directly affect the rate of reaction The rate of reaction is influenced by
Time does not affect the rate of a reaction Hence in the rate law expression r a t e k A n there is no term for time Nature of the reactants Concentrations surface area exposed and How does a decrease in the reactant concentration affect the reaction rate of a chemical reaction a The reaction rate does not change b The reaction rate increases c The reaction rate

Question Video Determining The Factor That Does Not Affect The Rate Of
https://media.nagwa.com/480109852128/en/thumbnail_l.jpeg

Collision Theory
https://www.chemistrystudent.com/images/ASPhysical/kinetics/collisiontheory5.png
Which Of The Following Does Not Affect The Rate Of Chemical Reaction - Apr 7 2019 nbsp 0183 32 The reaction CH3 3COOC CH3 3 g 2CH3COOCH3 g C2H6 g is carried out at constant volume with P0