Stoichiometry Mass Mass Problems Worksheet Answers STOICHIOMETRY WORKSHEET 1 MASS MASS 1 Determine the mass of lithium hydroxide produced when 0 38 grams of lithium nitride reacts with water according to the following unbalanced chemical equation Li3N s H2O l 174 NH3 g LiOH aq 2
a What mass of iron is needed to react with 16 0 grams of sulfur b How many grams of FeS are produced Solution to a 1 Determine moles of sulfur 16 0 g 256 52 g mol 0 0623733 mol S 8 2 Ratio and proportion S 8 to Fe is 1 8 There are four steps involved in solving these problems Make sure you are working with a properly balanced chemical equation Convert grams of the substance given in the problem to moles
Stoichiometry Mass Mass Problems Worksheet Answers
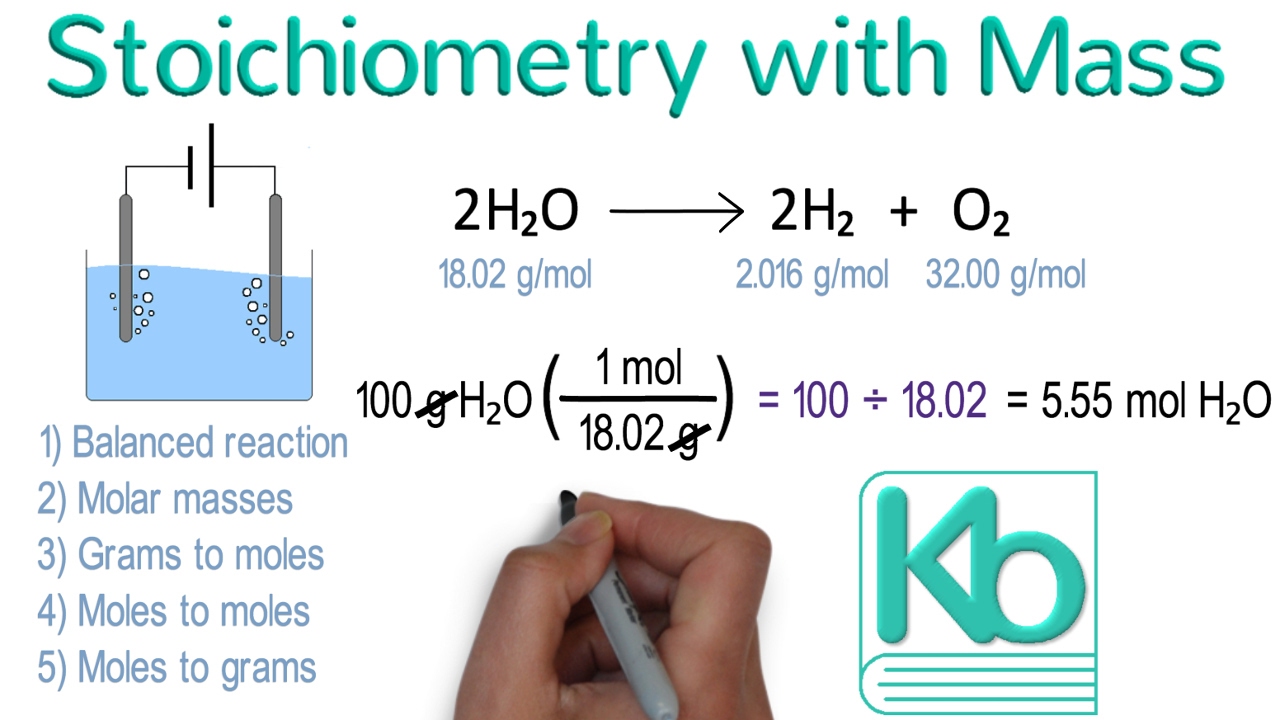
Stoichiometry Mass Mass Problems Worksheet Answers
https://i.ytimg.com/vi/BZuS3Agn4pI/maxresdefault.jpg

Examples Of Mass To Mass Stoichiometry Problems YouTube
https://i.ytimg.com/vi/BMPu3a9K0Zo/maxresdefault.jpg
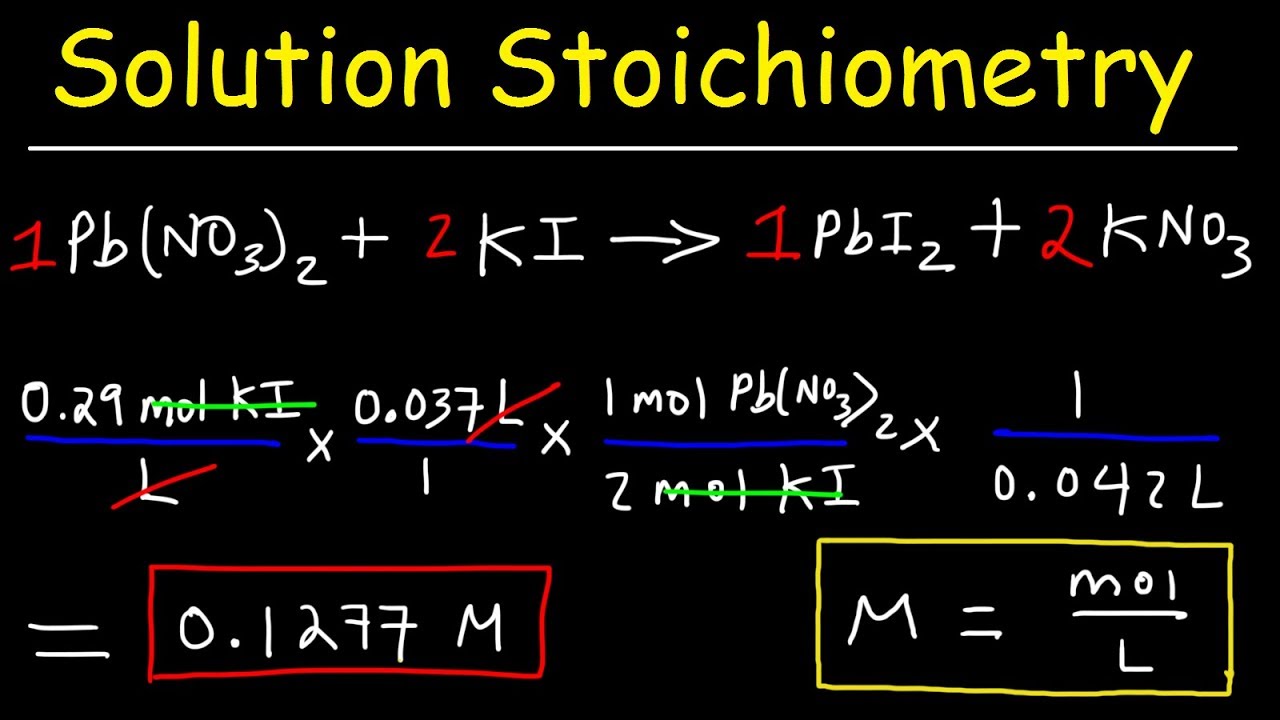
Solution Stoichiometry Finding Molarity Mass Volume YouTube
https://i.ytimg.com/vi/Ab3wfKjaWWQ/maxresdefault.jpg
This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given Sep 21 2022 nbsp 0183 32 Mass mass calculations involve converting the mass of a reactant to moles of reactant then using mole ratios to determine moles of product which can then be converted to mass of product
Stoichiometry Worksheet 1 Answers 1 Given the following equation 2 C 4H 10 13 O 2 gt 8 CO 2 10 H 2O show what the following molar ratios should be a C 4H 10 O 2 b O 2 CO 2 c O 2 H 2O d C 4H 10 CO 2 e C 4H 10 H 2O 2 Given the following equation 2 KClO 3 gt 2 KCl 3 O 2 a How many moles of O 2 can be produced by Calculate the mass of MnO 2 needed to produce 25 0g of Cl 2 b What mass of MnCl 2 is produced when 0 091g of Cl 2 is generated
More picture related to Stoichiometry Mass Mass Problems Worksheet Answers
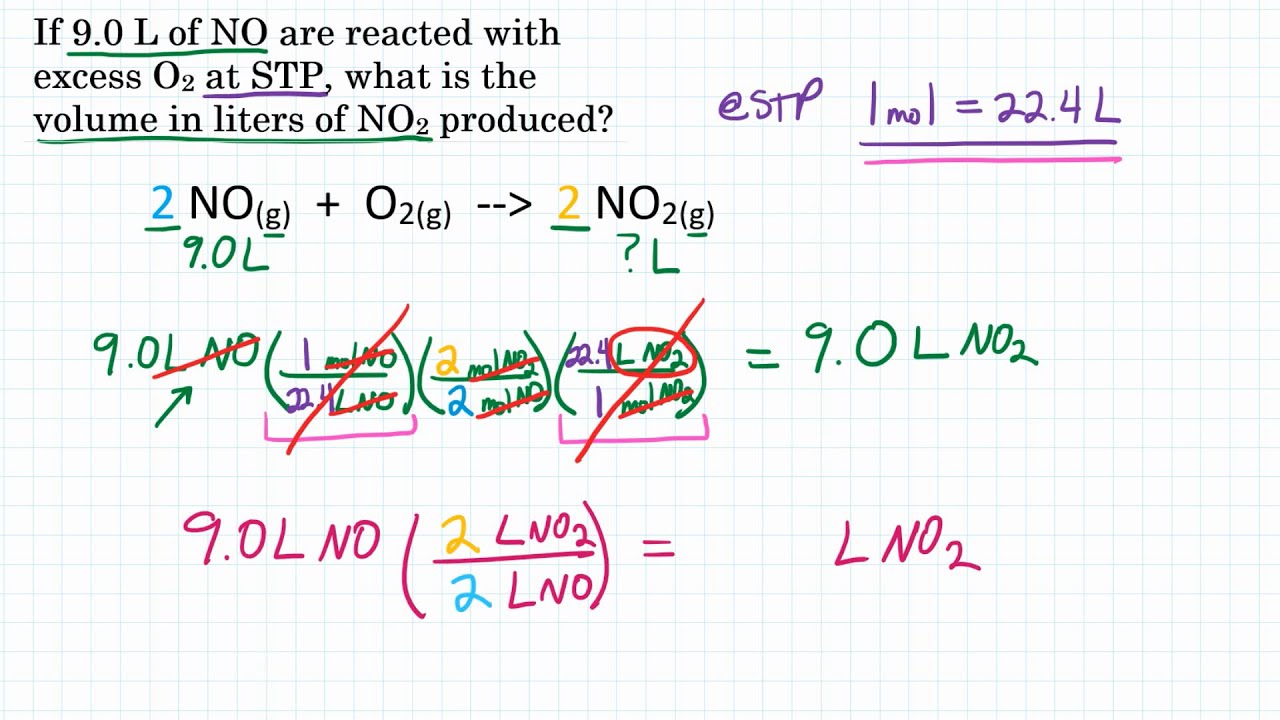
Ideal Gas Stoichiometry Volume To Volume Practice 1 YouTube
https://i.ytimg.com/vi/auF_HSWv0nE/maxresdefault.jpg

Stoichiometry With Mass YouTube
https://i.ytimg.com/vi/C8S11U6VnBQ/maxresdefault.jpg

Stoichiometry Mass To Volume YouTube
https://i.ytimg.com/vi/opb1Z75rcWI/maxresdefault.jpg
Stoichiometry WorkSheet 1 Worked Solutions Answer the following questions on your own paper Show all work Circle the final answer giving units and the correct number of significant figures 1 Based on the following equation how many moles of each product are produced when 5 9 moles of Zn OH 2 are reacted with H 3 PO 4 You need Stoichiometry with Solutions 1 H3PO4 3 NaOH gt Na3PO4 3 H2O How much 0 20 M H3PO4 is needed to react with 100 ml of 0 10 M NaOH
[desc-10] [desc-11]
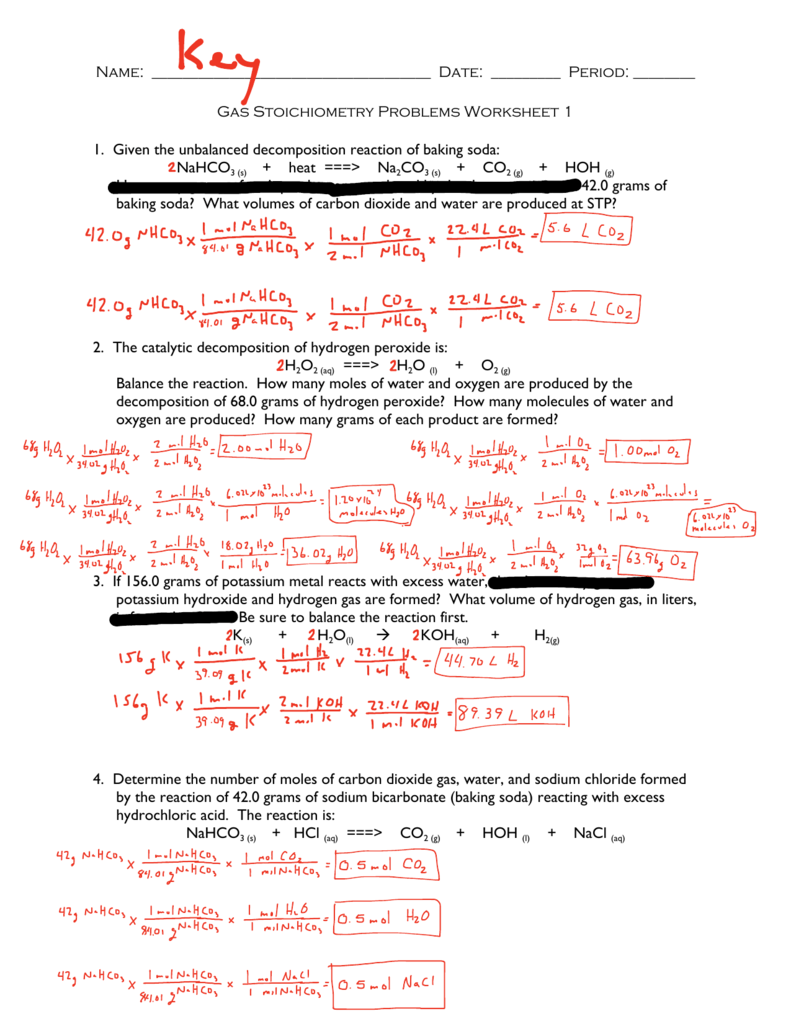
Gas Stoichiometry Worksheet Answer Key
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png
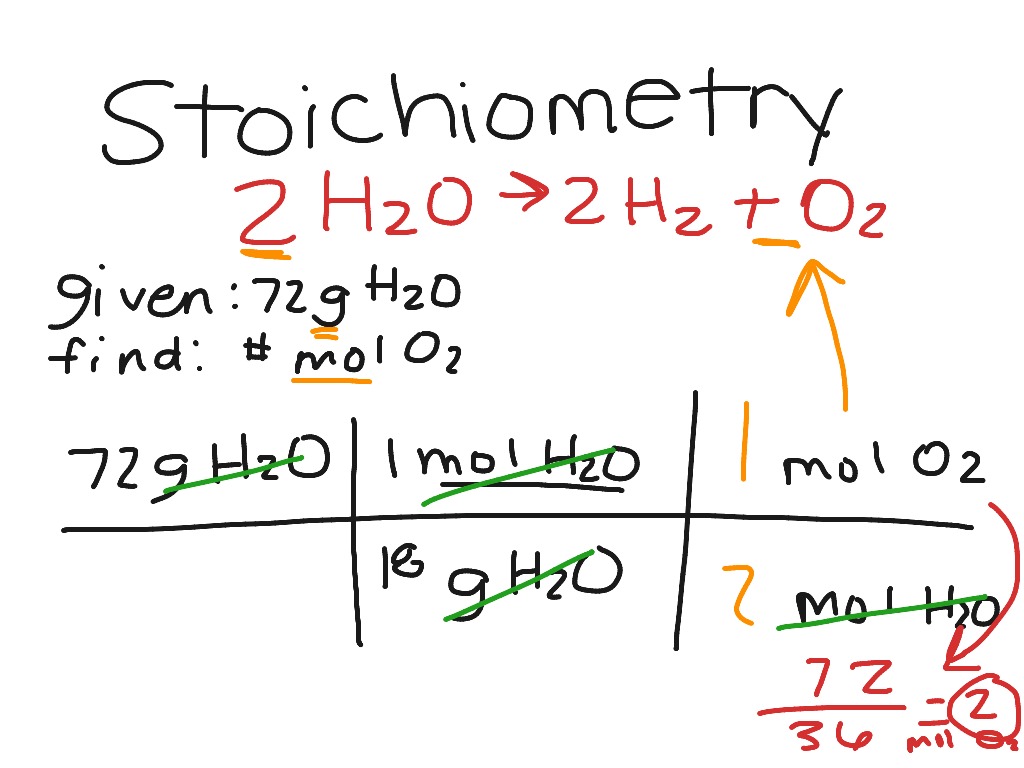
Stoichiometry Problem Type 3 Mass To Mol Science Chemistry ShowMe
https://showme0-9071.kxcdn.com/files/160329/pictures/thumbs/633496/last_thumb1358607525.jpg
Stoichiometry Mass Mass Problems Worksheet Answers - Sep 21 2022 nbsp 0183 32 Mass mass calculations involve converting the mass of a reactant to moles of reactant then using mole ratios to determine moles of product which can then be converted to mass of product