Molarity By Dilution Worksheet Answers PROBLEM 6 1 1 4 Determine the molarity of each of the following solutions 1 457 mol KCl in 1 500 L of solution 0 515 g of H 2 SO 4 in 1 00 L of solution 20 54 g of Al NO 3 3 in 1575 mL of solution 2 76 kg of CuSO 4 5H 2 O in 1 45 L of solution 0 005653 mol of Br 2 in 10 00 mL of solution
Solutions to the Molarity Practice Worksheet For the first five problems you need to use the equation that says that the molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution In this problem simply solve using the molarity equation to find that the concentration of the solution is 10 M PROBLEM 6 1 5 6 1 5 Calculate the number of moles and the mass of the solute in each of the following solutions a 2 00 L of 18 5 M H 2 SO 4 concentrated sulfuric acid b 100 0 mL of 3 8 10 5 M NaCN the minimum lethal concentration of sodium cyanide in blood serum c 5 50 L of 13 3 M H 2 CO the formaldehyde used to fix
Molarity By Dilution Worksheet Answers

Molarity By Dilution Worksheet Answers
https://s3.studylib.net/store/data/009088253_1-9e7fcf7278fc15f9654bf149bf8282da.png

Molarity Dilution Calculations YouTube
https://i.ytimg.com/vi/61x0GvGzp2U/maxresdefault.jpg

Molarity Practice Problems YouTube
https://i.ytimg.com/vi/FmCwGMWMr8U/maxresdefault.jpg
Molarity calculations Google Classroom You might need Calculator A 0 674 M cobalt II chloride CoCl 2 solution is prepared with a total volume of 0 0750 L The molecular weight of CoCl 2 is 128 9 g mol Molarity Review Problems 1 What is the molarity of a solution in which 0 45 grams of sodium nitrate are dissolved in Dilutions Worksheet Solutions 1 If I add 25 mL of water to 125 mL of a 0 15 M NaOH solution what will the molarity of The answer 700 mL Titrations Practice Worksheet Solutions 1 If it takes 54 mL of 0 1 M NaOH to
This worksheet will help you to practice the three types of dilution problems These are the same directions that were on Dilutions practice Sheet 1 TYPE 1 MIXING T Add the amount of liters together Step 4 Determine the new molarity by dividing the total moles by the total liters Example 600 mL of a 3 M sugar water solution Dilutions worksheet MOLARITY EXTRA PRACTICE WORKSHEET 1 What is the molarity of 2 0 L of solution made from 2 4 moles of NaCl and water 2 What is the molarity of 500 mL of solution made from 0 70 moles of LiCl and water 3 What is the molarity of 100 mL of solution made from 0 45 g of NaOH and water
More picture related to Molarity By Dilution Worksheet Answers

Solutions Worksheet Molarity And Dilution Problems Answer Key
https://www.problemsworksheets.com/wp-content/uploads/2023/03/molarity-worksheet-answer-key.png

Molarity Dilution Practice Problem 3 YouTube
https://i.ytimg.com/vi/SgPxDRixM3Q/maxresdefault.jpg

Dilutions Worksheet
https://s3.studylib.net/store/data/007901743_2-beb2c916a39d4e2de6bca6bebab1c58b.png
Chemistry questions and answers Molarity and Dilutions Tutorial Worksheet Part 1 Forming Aqueous Solutions from Ionic Solids Many ionic compounds are soluble in water When a soluble ionic compound dissolves in water the cations and anions are separated In the solution the cations and anions are surrounded by water molecules Placing the proper values into the dilution equation gives 2 500 mol L 100 0 mL 0 5500 mol L x x 454 5 mL Sometimes the problem might ask how much more water must be added In this last case the answer is 454 5 100 0 354 5 mL Go ahead and answer the question if your teacher asks it but it is bad technique in the
Molarity amp Dilution Practice Problems Answers Determine the molarity of a solution containing 2 mol of KI in 140 mL total volume of solution ANS 17 M KI What is the concentration of a solution of NaCl if 40 mL of a 2 M NaCl solution is diluted to a total volume of 500 mL ANS 0 M NaCl Dilutions Worksheet Solutions 1 If I add 25 mL of water to 125 mL of a 0 15 M NaOH solution what will the molarity of the diluted solution be M1V1 M2V2 0 15 M 125 mL x 150 mL x 0 125 M 2 If I add water to 100 mL of a 0 15 M NaOH solution until the final volume is 150 mL what will the molarity of the diluted solution be

7 Best Images Of Molarity Worksheet With Answers Molality And
http://www.worksheeto.com/postpic/2011/05/molarity-problems-worksheet-answer-key_224207.png
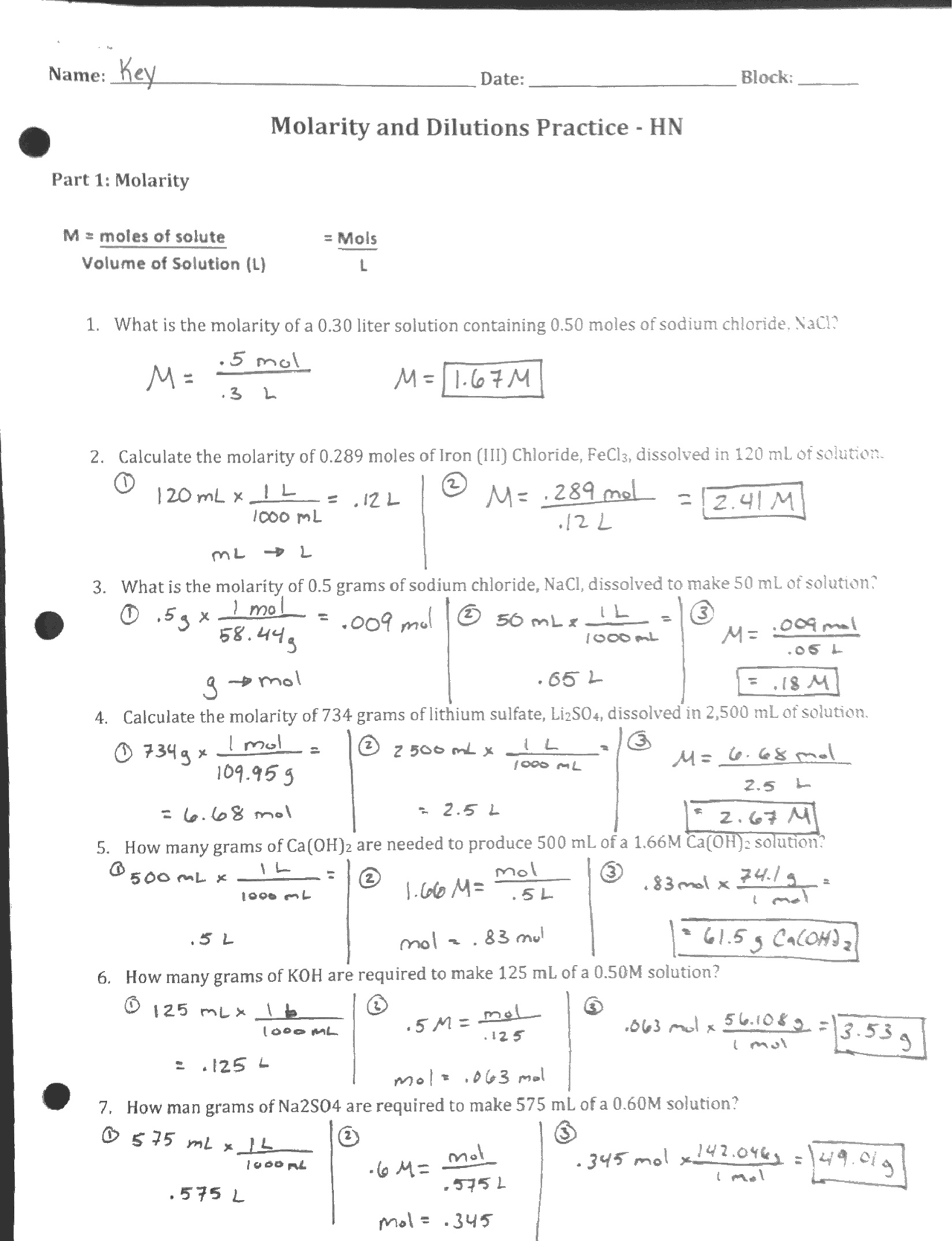
Molarity And Dilutions Practice Worksheet Key Docsity
https://static.docsity.com/documents_first_pages/2021/04/20/6001daadf7683f72f8dab401484a4713.png?v=1651987055
Molarity By Dilution Worksheet Answers - Solution Calculate the molarity of sulfate SO42 in this solution First find moles of sulfate from the grams given Then divide by the volume in liters to get the molarity 0 001036 M 3 What mass of solute is contained in 149 mL of a 0 728 M ammonium nitrate NH4NO3 solution Use the volume and molarity given to find moles of the solute