Periodic Trends Worksheet Answers Chemistry Periodic Trends Worksheet A nswer Key 1 Circle the element with the largest atomic radius and put a square around the element with the smallest atomic radius Cu K Ni Br Largest K Smallest Br Explain why you made these choices Atomic radius decreases as you go left to right across a
Worksheet Periodic Trends 1 ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius a Li C F b Li Na K c Ge P O d C N Al e Al Cl Ga 2 IONIC RADIUS For each of the following sets of ions rank them from smallest to largest ionic radius a Mg 2 Si 4 S 2 b Mg 2 Ca 2 3 What trends do you notice for the atomic radii of Period 3 The atomic radius gets smaller as atomic number increases 4 Explain why this trend occurs Going from left to right across a period the size gets smaller Electrons are in the same energy level but there is more nuclear charge more protons The increased charge pulls electrons
Periodic Trends Worksheet Answers Chemistry
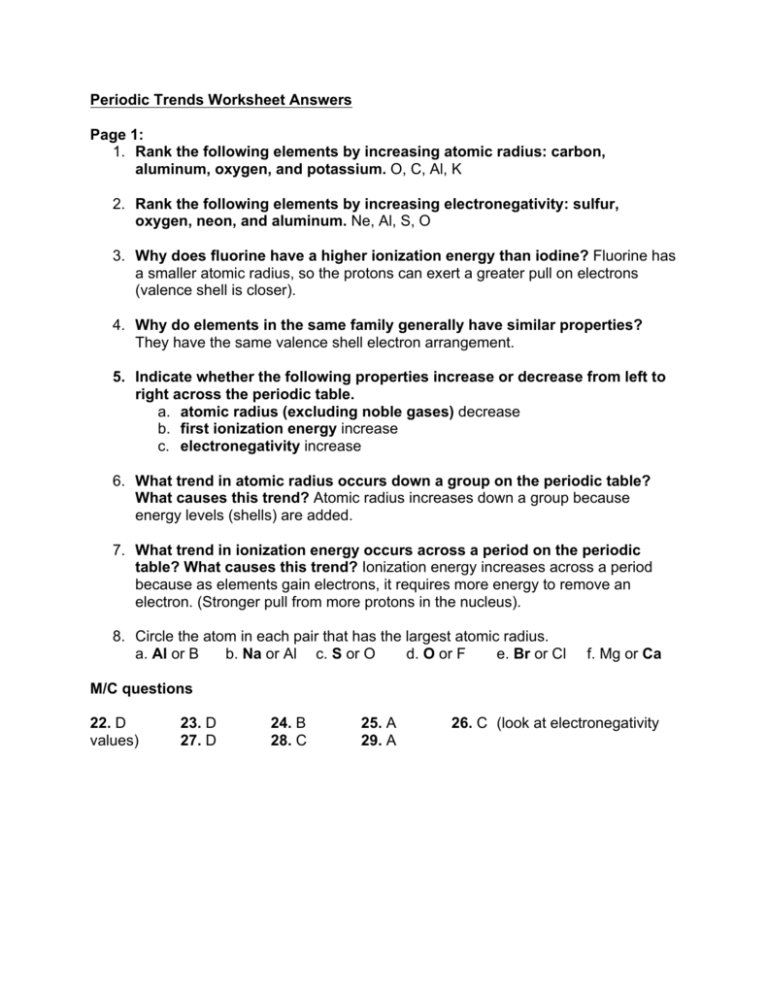
Periodic Trends Worksheet Answers Chemistry
https://s3.studylib.net/store/data/008677423_1-39c05b4c6f127791faf0d05b4752e9dc-768x994.png

Periodic Trends Worksheet 1 Answers Periodic Trends Worksheet Use The
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/c96bf05ef93ed3b824c5155f1f298331/thumb_1200_1553.png

50 Periodic Trends Worksheet Answers
https://chessmuseum.org/wp-content/uploads/2019/10/periodic-trends-worksheet-answers-awesome-periodic-trends-worksheet-answers-chemistry-awesome-6-3-of-periodic-trends-worksheet-answers.jpg
It provides the answers to questions about classifying elements as metals nonmetals or metalloids It also answers questions about trends in atomic radius ionization energy and electronegativity across the periodic table including ordering elements by these properties and defining each term For each of the following oxides indicate whether it is ionic or molecular and whether it is acidic or basic Then write a balanced equation for the reaction expected between each oxide and water
May 25 2020 nbsp 0183 32 Name 1 Label the periodic table above according to the periodic trends ionization energy electron affinity and atomic radius Increase gt Decrease or Decrease gt Increase 2 List the following elements in order of increasing atomic radius carbon aluminum oxygen and potassium Which of the following in each pair has the larger atomic radius II Which of the following in each pair has the larger electronegativity 15 What do you notice about the answers in I vs the answers in II Why is this true Opposite larger radius equals less attraction for e III
More picture related to Periodic Trends Worksheet Answers Chemistry

Worksheet Periodic Trends Answers Chemistry I 2005 Worksheet 4 3
https://i.pinimg.com/originals/cf/e3/b1/cfe3b10c7b26b52320ca13466b2e0ce9.jpg
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Getting To Know The Periodic Table Worksheet Answers Brokeasshome
https://www.thoughtco.com/thmb/dbJzy9sDxrpmp5GqnuSEfzwdFKU=/1333x1000/smart/filters:no_upscale()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png

Periodic Table Riddles Unique Periodic Trends Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/periodic-table-riddles-unique-periodic-trends-worksheet.png
Mar 13 2023 nbsp 0183 32 Knowing the trends in atomic and ionic sizes ionization energies and electron affinities aids in understanding chemical behavior and the nature of chemical bonds Be able to predict differences in ionization energy and electron affinity among elements Periodic Trends Worksheet Rank the following elements by increasing atomic radius carbon aluminum oxygen potassium Rank the following elements by increasing electronegativity sulfur oxygen neon aluminum What is the difference between electron affinity and ionization energy Cc Why does fluorine have a higher ionization energy than iodine
If you are struggling with problems concerning periodic trends we have prepared dozens of interesting periodic trends worksheets for extra practice As atomic number increases atomic radius decreases The increased core charge due to the increased number of protons in the nucleus draws the electrons closer Yes As the number of protons increases across period 2 the atomic radius will decrease

Periodic Trends Worksheet Answers Chemistry
https://briefencounters.ca/wp-content/uploads/2018/11/periodic-trends-worksheet-answers-chemistry-or-periodic-table-worksheet-2-answers-choice-image-periodic-table-of-of-periodic-trends-worksheet-answers-chemistry.png

8 Pics Trends Of The Periodic Table Worksheet Part 1 Answer Key And
https://alquilercastilloshinchables.info/wp-content/uploads/2020/06/Periodic-Trends-Worksheet.png
Periodic Trends Worksheet Answers Chemistry - Aug 30 2016 nbsp 0183 32 Explain why you made these choices All of the elements are in the same period The trend in atomic radius as you go across a period is DECREASING Therefore the element on the far left K is the largest and the element on the far right Br is the smallest Cu K Ni Br a