Periodic Trends Practice Worksheet Answers 3 What trends do you notice for the atomic radii of Period 3 The atomic radius gets smaller as atomic number increases 4 Explain why this trend occurs Going from left to right across a period the size gets smaller Electrons are in the same energy level but there is more nuclear charge more protons The increased charge pulls electrons
Answers for Comparing Tendencies to Gain Electrons Here are answers to the exercises above Li C N Li has the least tendency to gain electrons because it has the lowest effective nuclear charge and all use the same number of energy levels What trend in ionization energy occurs across a period on the periodic table What causes this trend
Periodic Trends Practice Worksheet Answers

Periodic Trends Practice Worksheet Answers
https://s1.studyres.com/store/data/000966540_1-df138a9992cb3d2189ffe4335f45797e.png
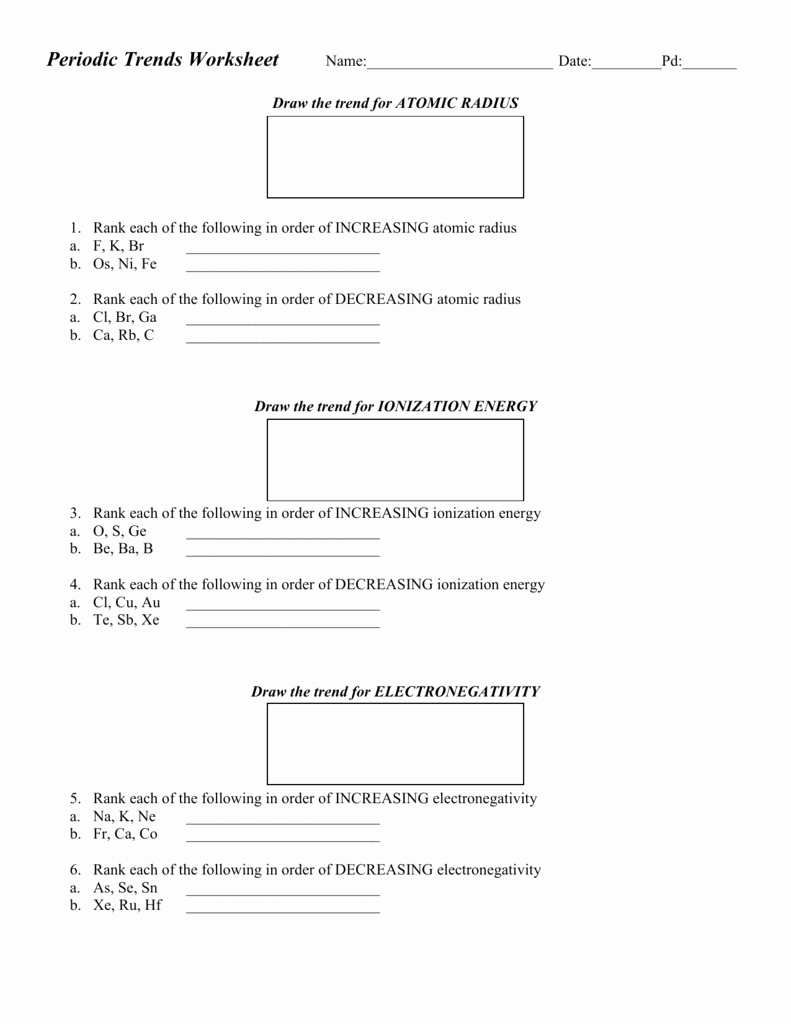
50 Periodic Trends Practice Worksheet Answers
https://chessmuseum.org/wp-content/uploads/2019/10/periodic-trends-practice-worksheet-answers-lovely-periodic-trends-hw-key-on-last-pages-of-periodic-trends-practice-worksheet-answers.png
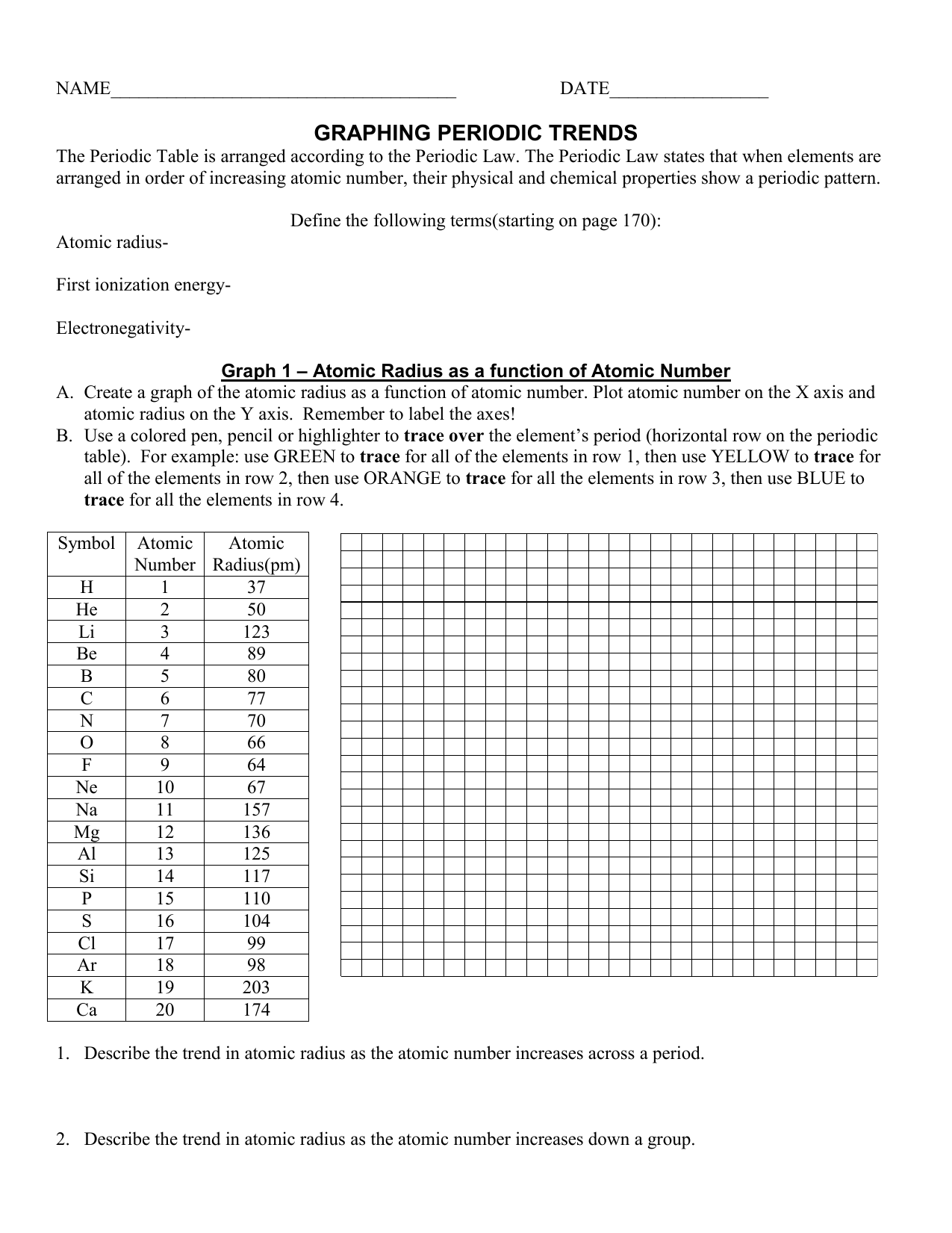
Periodic Trends Worksheet Answer Key
https://s2.studylib.net/store/data/025642916_1-c94db2359aa7e3e6b76578c306ee02ba.png
Based on the concept of periodic trends answer the following questions for these atoms Au Zn S Si Be able to defend your answers a Which element has the highest electronegativity b Which element has the most metallic character c Mar 13 2023 nbsp 0183 32 Know periodic trends of atomic size ionic size ionization energy and electron affinity Understand the reasons for metallic nonmetallic and metalloid character Understand why some acids dissolve in water to make acidic solution while others dissolve in water to make basic solutions
EXTRA PRACTICE Periodic Trends Practice 2 WS ANSWER KEY copy Extra Practice Periodic Trends 1 ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius a Li C b Li Na K 165 L k e Al Cl Ga C I 2 Periodic Trends Practice Worksheet Use the periodic table and your knowledge of the periodic trends to answer the following questions Identify each element as a metal
More picture related to Periodic Trends Practice Worksheet Answers

Periodic Table Worksheet Answers
https://briefencounters.ca/wp-content/uploads/2018/11/periodic-table-worksheet-answers-or-47-inspirational-collection-periodic-trends-practice-worksheet-of-periodic-table-worksheet-answers.jpg
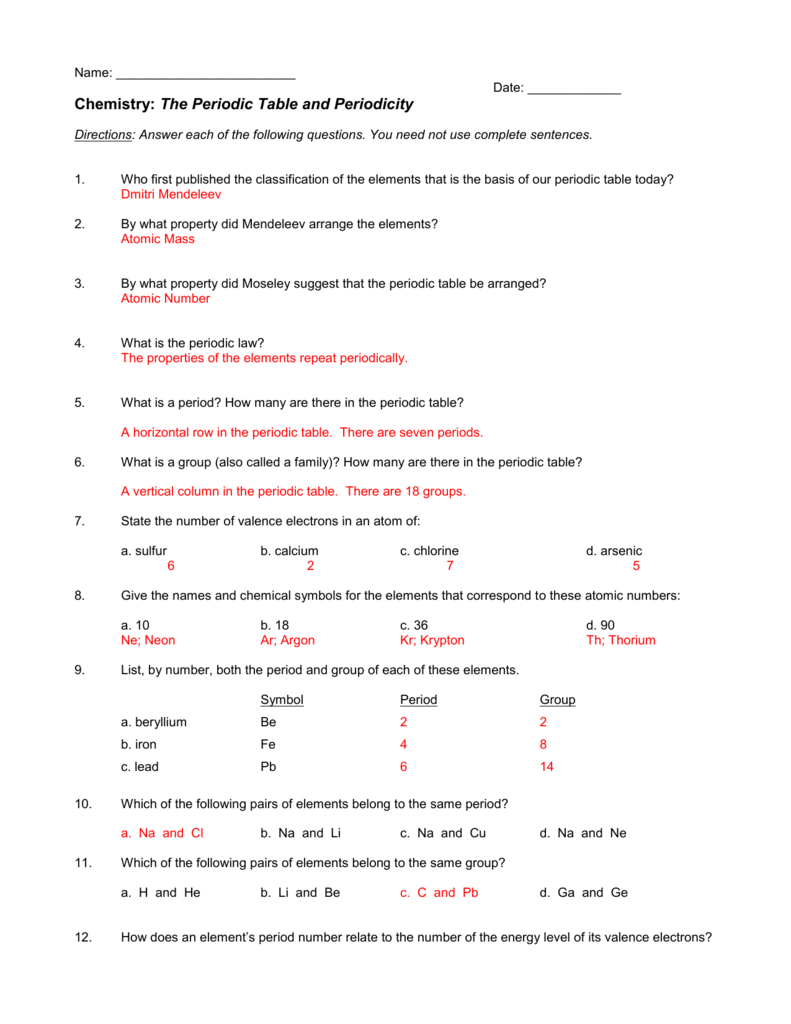
Periodic Table Review Questions 300 Key
https://s3.studylib.net/store/data/009193726_1-f0f6b9d57f7da42b36592f2cef15165f.png

Periodic Trends Worksheet Answer Key
https://excelguider.com/wp-content/uploads/2019/07/periodic-trends-worksheet-answers-pogil-p90x-worksheets-monohybrid-as-well-as-periodic-trends-worksheet-answers-pogil.jpg
This online quiz is intended to give you extra practice in identifying different periodic trends such as atomic radius ionization energy and electron affinity This quiz aligns with the following NGSS standard s HS PS1 1 HS PS1 2 Created Date 11 3 2015 3 20 46 PM
A Explain why you made these choices All of the elements are in the same period The trend in ionization energy as you go across a period is INCREASING Therefore the element on the far left K has the lowest ionization energy and the element on the far right Br has the highest ionization energy 3 Apr 28 2024 nbsp 0183 32 Would you expect B Al and Ga to act as a triad Justify your answers Despite the fact that Dobereiner Newlands Meyer and Mendeleev all contributed to the development of the modern periodic table Mendeleev is credited with its origin Why was Mendeleev s periodic table accepted so rapidly
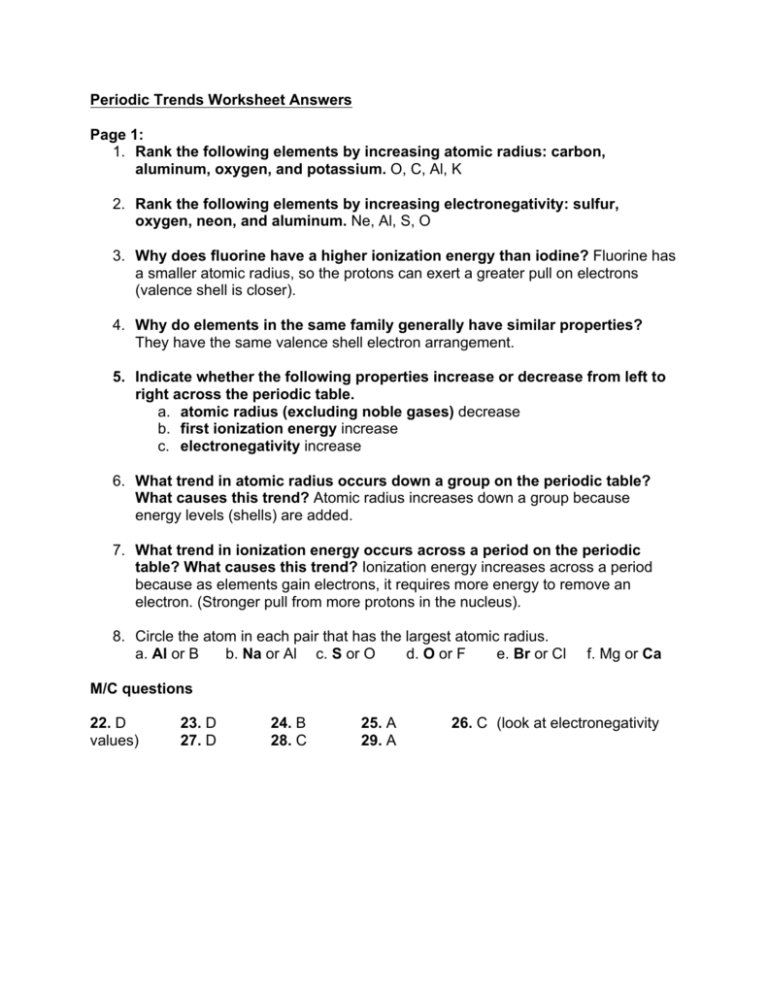
Periodic Trends Worksheet Answers Page 1 1 Rank The Following
https://s3.studylib.net/store/data/008677423_1-39c05b4c6f127791faf0d05b4752e9dc-768x994.png

Chapter 6 The Periodic Table Practice Problems Worksheet Answers
https://i2.wp.com/s3.studylib.net/store/data/008128857_1-57499ea3e40ce485a38b6dea1db20a83.png?resize=618%2C800&ssl=1
Periodic Trends Practice Worksheet Answers - Periodic Trends Practice Worksheet Answer Key 1 Free download as PDF File pdf Text File txt or read online for free Atomic size increases down a group and decreases left to right in a period