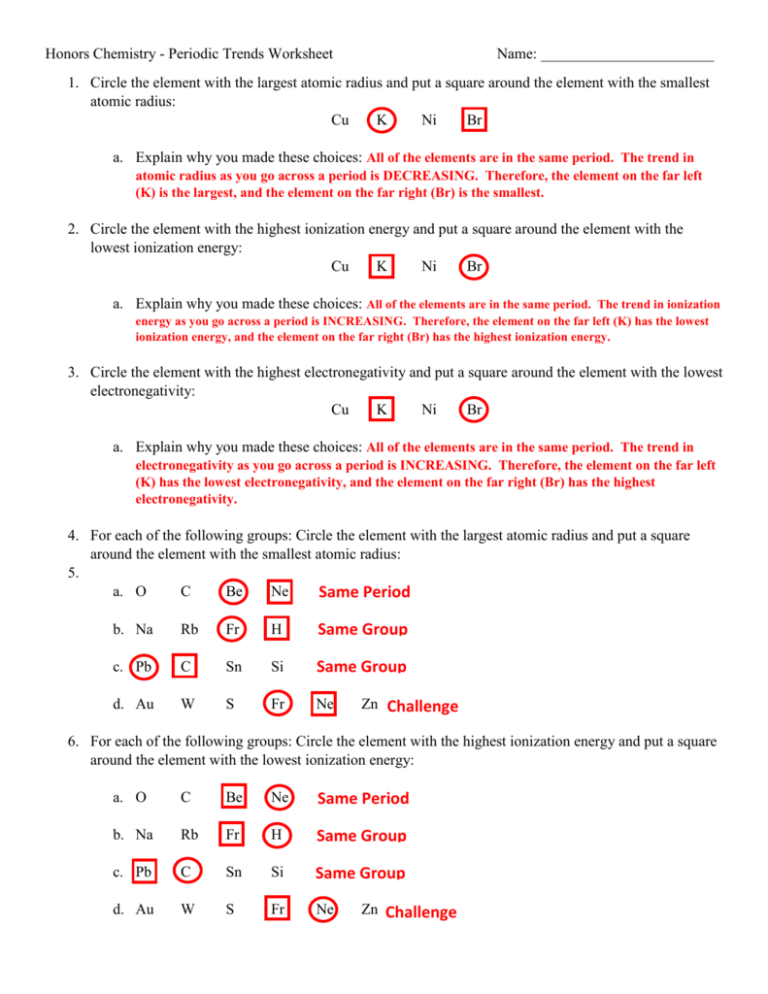
Periodic Trends Ionization Energy Worksheet Answers A Atomic Radius Excluding noble gases B First ionization energy C Electronegativity A Decrease B Increase C Increase What trend in atomic radius occurs down a group on the periodic table What causes this trend Increases down group because energy level shells are added
Periodic trends Google Classroom You might need Periodic table The first two ionization energies for beryllium are shown below Be g Be g e Be g Be 2 g e I 1 900 kJ mol I 2 1757 kJ mol Which of the following identifies the most probable value for the third ionization energy for Be and provides the 4 Give your best and most concise explanation of the following trends a There is a general trend in atomic radius across the table it decreases as you go from left to right across a period b There is a general trend in atomic radius down the table it increases as you go down a group 5 Examine the charts above
Periodic Trends Ionization Energy Worksheet Answers

Periodic Trends Ionization Energy Worksheet Answers
https://i0.wp.com/www.energyworksheet.com/wp-content/uploads/2022/10/1st-ionization-energy-trend-on-periodic-table-review-home-decor-2.png?resize=768%2C994&ssl=1

Periodic Trends In Ionization Energy Chemistry Socratic
https://useruploads.socratic.org/H7ptw5aRSadPRg7SOPwa_ptable orbitals ionization energy.png

Periodic Trends Worksheet 1 Answers Periodic Trends Worksheet Use The
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/c96bf05ef93ed3b824c5155f1f298331/thumb_1200_1553.png
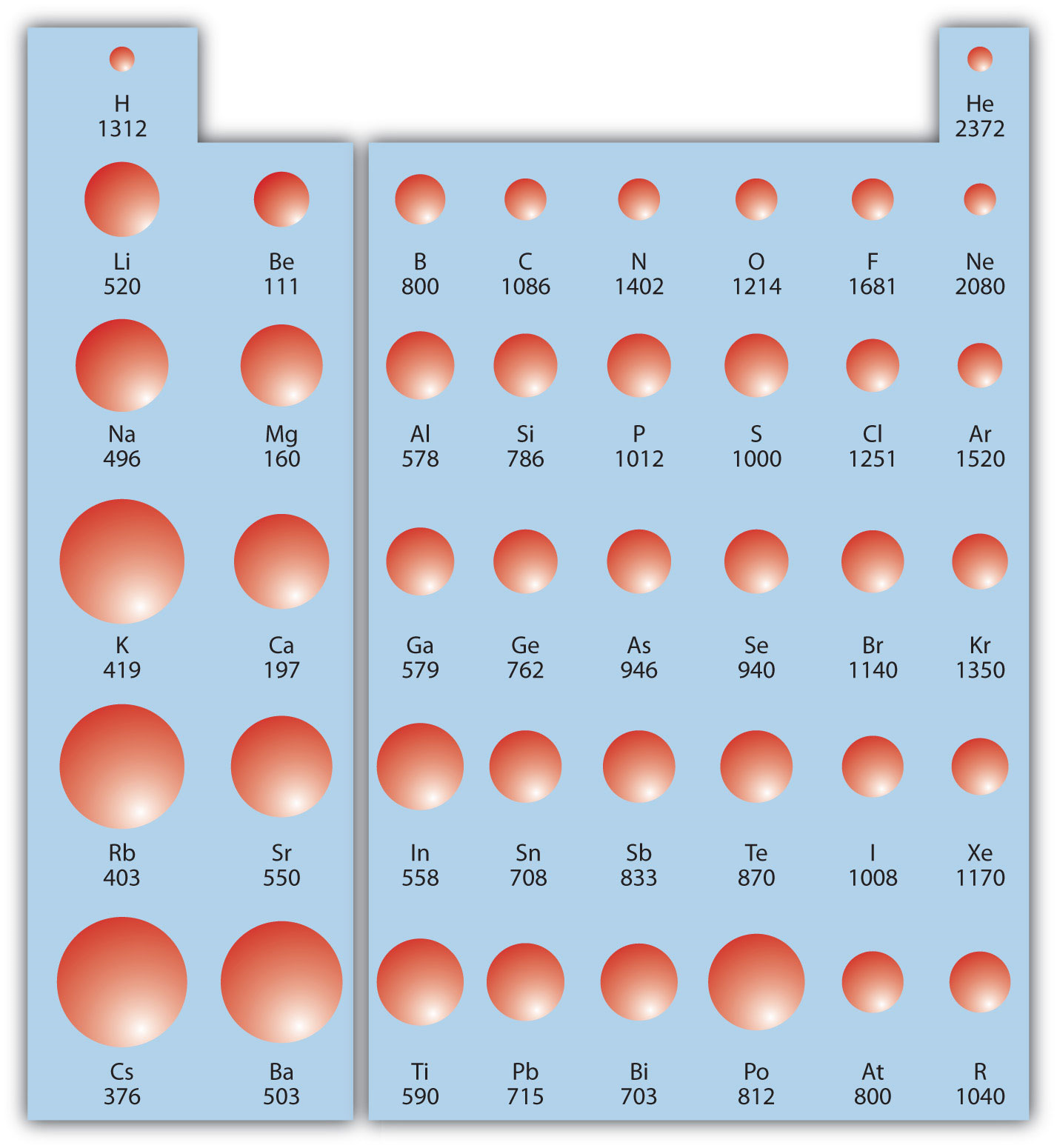
Ionization Energy Trends Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase Conceptually ionization energy is the opposite of electronegativity Answer C Oxygen O Explanation Periodic trends indicate that atomic radius increases up a group and from left to right across a period Go to the Periodic Table Live at www chemeddl to be able to choose which elements groups and periods will be graphed to answer this worksheet Select any main group elements period excluding 6 and 7 On the graph to the right of Matsumoto P S 2005 Trends in ionization energy of transition metal elements Journal of Chemical
Answers to Comparing Ionization Energies Here are answers to the exercises above Mg Si S All are in the same period and use the same number of energy levels Mg has the lowest I E because it has the lowest effective nuclear charge S has the highest I E because it has the highest effective nuclear charge Use the periodic table charts and your knowledge of periodic trends to answer the following questions Atomic Radius 1 Which atom in each pair has the larger atomic radius Circle your answer a Li or K b Ca or Ni c Ga or B d O or C e Cl or Br f Be or Ba g Si or S
More picture related to Periodic Trends Ionization Energy Worksheet Answers

Electronegativity Chart Template Ionization Energy Google With
https://i.pinimg.com/originals/3d/fd/84/3dfd845f77ee1114393032a45f1cb7fb.png
Periodic Trends Atomic Radius Worksheet Answers
https://s3.studylib.net/store/data/006773533_1-4885f6e36ae9fec4ec4b9a1b7b46d97f-768x994.png

Periodic Trends Notes And Worksheet Set Radius Electronegativity
https://i.pinimg.com/originals/95/66/39/95663994cfff60f789f735eaf2c39570.png
Worksheet 12 Periodic Trends A number of physical and chemical properties of elements can be predicted from their position in the Periodic Table Among these properties are Ionization Energy Electron Affinity and Atomic Ionic Radii These properties all involve the outer shell valence electrons as well as the inner shell shielding Periodic trends such as electronegativity electron affinity atomic and ionic radii and ionization energy can be understood in terms of Coulomb s law which is F q q r For example consider first ionization energy Coulomb s law tells us that the greater the nuclear charge q and the shorter the distance between the nucleus and the outermost electron r the stronger the
1 Ar 3s 23p 6 2 Po 6s 26p 4 3 Ga 4s 24p 1 What is the trend for atomic size as one proceeds down a family and explain the reason As you go down a family the atomic size increases b c more shells are filled increasing the distance between the outer shell to the nucleus Last updated September 09 2022 In this simulation students can investigate the periodic trends of atomic radius ionization energy and ionic radius By choosing elements from the periodic table atoms can be selected for a side by side comparison and analysis Students can also attempt to ionize an atom by removing its valence electrons
9 9 Periodic Trends Atomic Size Ionization Energy And Metallic
https://chem.libretexts.org/@api/deki/files/91280/Ionization_Energy_on_the_Periodic_Table.png?revision=1

Periodic Trends Ionization Energy Worksheet Answers
https://i0.wp.com/apps-dso.sws.iastate.edu/si/documentdb/fall_2011/CHEM_163_Windus___Bonaccorsi_emj_2011-12-07_18-57-05_3.jpg?resize=1040%2C1345&ssl=1
Periodic Trends Ionization Energy Worksheet Answers - Ionization Energy Trends Ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase Conceptually ionization energy is the opposite of electronegativity Answer C Oxygen O Explanation Periodic trends indicate that atomic radius increases up a group and from left to right across a period