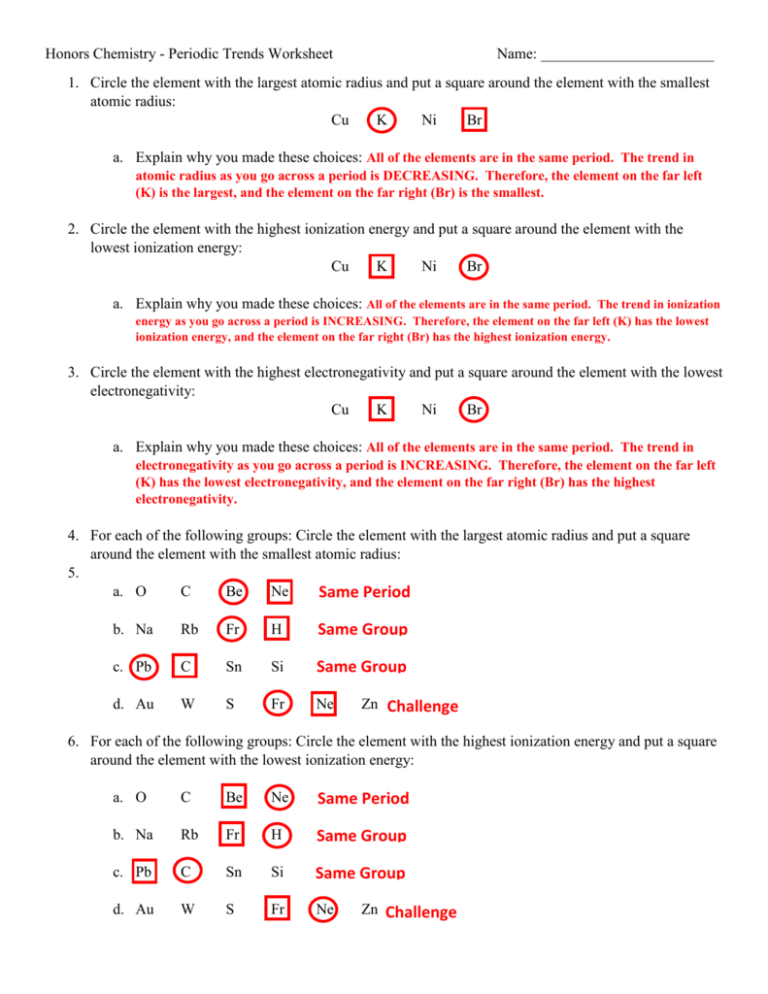
Periodic Trends Ionization Energy Worksheet Answer Key What trend in ionization energy occurs across a period on the periodic table What causes this trend
Predict Ionization energy IE is the energy required to remove an electron from an atom As atomic radius increases the valence electrons get farther from the nucleus How do you think an atom s size willaffect its ability to hold on to its valence electrons IONIZATION ENERGY For each of the following sets of atoms rank them from lowest to highest ionization energy a Mg b Mg Ca Ba d Ba cu Ne 4 ELECTRONEGATIVITY For each of the following sets of atoms rank them from lowest to highest electronegativity a Li C N c Si P 0 d K Mg K P doe Title Created Date
Periodic Trends Ionization Energy Worksheet Answer Key

Periodic Trends Ionization Energy Worksheet Answer Key
https://img.homeworklib.com/questions/51634c20-3da1-11ea-bac0-899474208981.png?x-oss-process=image/resize,w_560

Periodic Trends In Ionization Energy Chemistry Socratic
https://useruploads.socratic.org/H7ptw5aRSadPRg7SOPwa_ptable orbitals ionization energy.png

Periodic Trends Worksheet 1 Answers Periodic Trends Worksheet Use The
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/c96bf05ef93ed3b824c5155f1f298331/thumb_1200_1553.png
Periodic trends Ionization energy answers 1 What is the atomic number The number of protons found in an element 2 What is the ionization energy of an element It is the minimal energy needed to remove an electron from the outer shell of a neutral atom in a gaseous state 3 Worksheet P riodic Trends I Which statement best describes Group 2 elements as they are considered in order from top to bottom of the Periodic Table A The number of principal energy levels increases and the number of valence electrons increases B The numbcr of principal energy levels increases and
Unit 3 Periodic Trends IE E neg 1 1 Circle the atom in each pair that has the greater ionization energy a Li or Be 2 For which of the properties does Li have a larger value than potassium Use the periodic table not the tables or charts in your text a first ionization energy b atomic radius c ionic radius d number of protons See end of Answer Key for answers to A D A Label the metals and the nonmetals B For the vertical arrow indicate the trend for atomic radius AR ionization energy IE and electron affinity EA by writing next to the arrow whether each property increases or decreases
More picture related to Periodic Trends Ionization Energy Worksheet Answer Key

Periodic Trends Notes And Worksheet Set Radius Electronegativity
https://i.pinimg.com/originals/95/66/39/95663994cfff60f789f735eaf2c39570.png

Electronegativity Chart Template Ionization Energy Google With
https://i.pinimg.com/originals/3d/fd/84/3dfd845f77ee1114393032a45f1cb7fb.png
Periodic Trends Atomic Radius Worksheet Answers
https://s3.studylib.net/store/data/006773533_1-4885f6e36ae9fec4ec4b9a1b7b46d97f-768x994.png
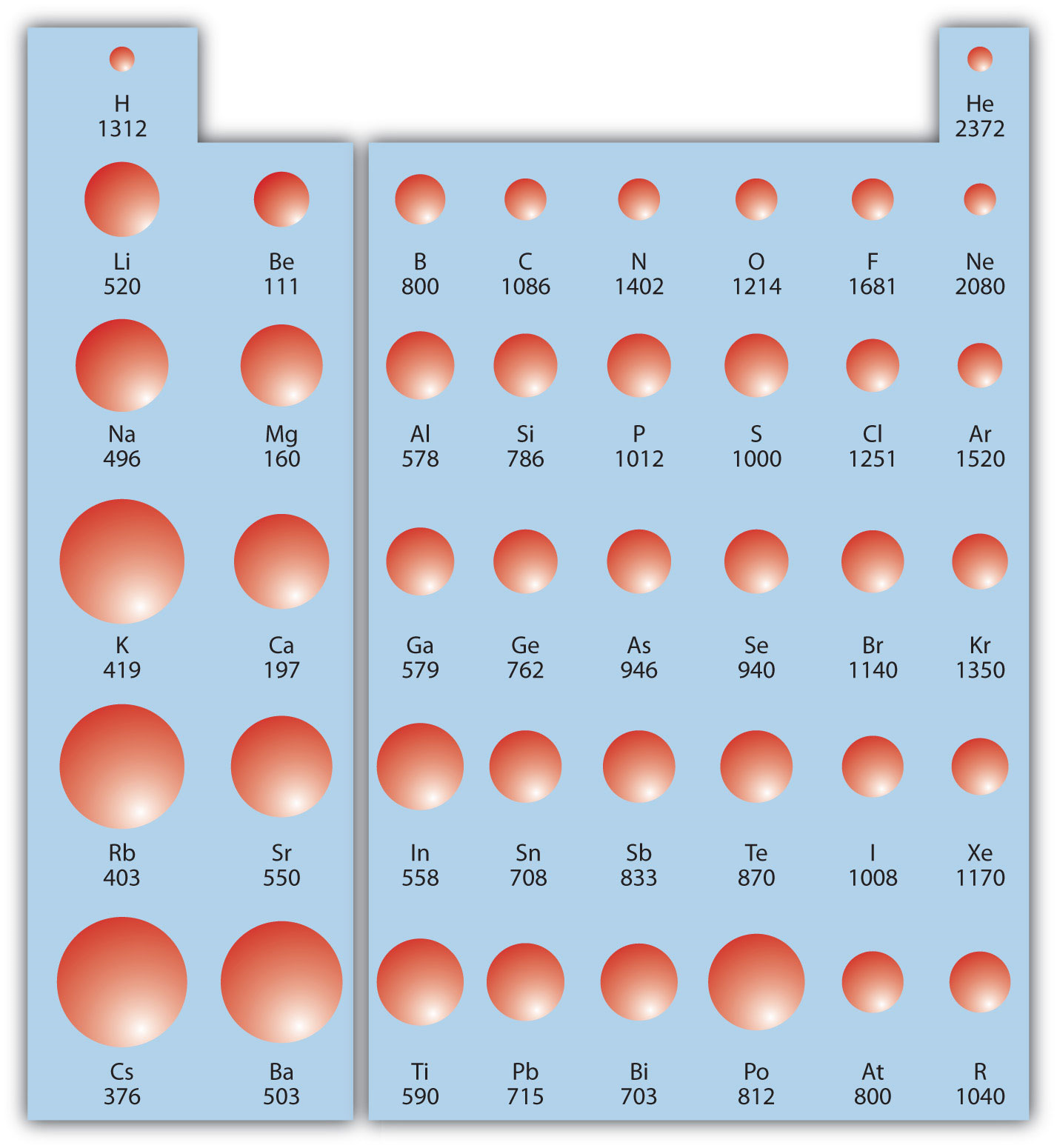
Worksheet Periodic Trends 1 ATOMIC RADIUS For each of the following sets of atoms rank the atoms from smallest to largest atomic radius a Li C F b Li Na K c Ge P O d C N Al e Al Cl Ga 2 IONIC RADIUS For each of the following sets of ions rank them from smallest to largest ionic radius a Mg 2 Si 4 S 2 b Mg 2 Ca 2 Mar 13 2023 nbsp 0183 32 Ionization Energy Ionization energy I is the energy required to remove an electron from a gaseous species The first ionization energy I 1 refers to removing one electron from a neutral atom X g rightarrow X g e nonumber with E I 1 gt 0
Tions about atomic size electronegativity ionization energies bonding solubility and reactivity In this activity you will look at a few periodic trends that can help you make those predictions Like most trends they are not perfect but useful just the same 1 Consider the data in Model 1 on the following page a What is the difference between electronegativity and ionization energy Answer ionization energy is the energy required to remove an electron whereas electronegativity is the affinity of an atom to a neighbouring atoms electrons
9 9 Periodic Trends Atomic Size Ionization Energy And Metallic
https://chem.libretexts.org/@api/deki/files/91280/Ionization_Energy_on_the_Periodic_Table.png?revision=1

Exceptions To The Ionization Energy Trend Wize University Chemistry
https://d3rw207pwvlq3a.cloudfront.net/attachments/000/122/231/images/img_poster.0000000.jpg?1615771880
Periodic Trends Ionization Energy Worksheet Answer Key - Table below gives the ionization energies for potassium bromine and calcium Identify which element is which from the data given Explain your answer in the space provided