Molecular And Empirical Formula Worksheet Write the empirical formula for the following compounds A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole What is the molecular formula of this compound
Empirical and Molecular Formulas Worksheet 1 What is the empirical formula for C8H18 2 What is the empirical formula for H2O 3 What is the empirical formula for C4H10 4 What is the empirical formula for C2H4O2 5 Is CO2 an empirical 5 D e t e rm i ne t he e m pi ri c a l a nd m ol e c ul a r form ul a of a c om pound c om pos e d of 18 24 g C a rbon 0 51 g H ydroge n a nd 16 91 g F l uori ne ha s a m ol a r m a s s 562 0 g m ol
Molecular And Empirical Formula Worksheet

Molecular And Empirical Formula Worksheet
https://i.ytimg.com/vi/0yp20L7_XzU/maxresdefault.jpg

Chemistry Made Simple Empirical Formula Worksheet For Practice Style
https://i2.wp.com/www.worksheeto.com/postpic/2012/08/molecular-and-empirical-formula-worksheet_224258.png
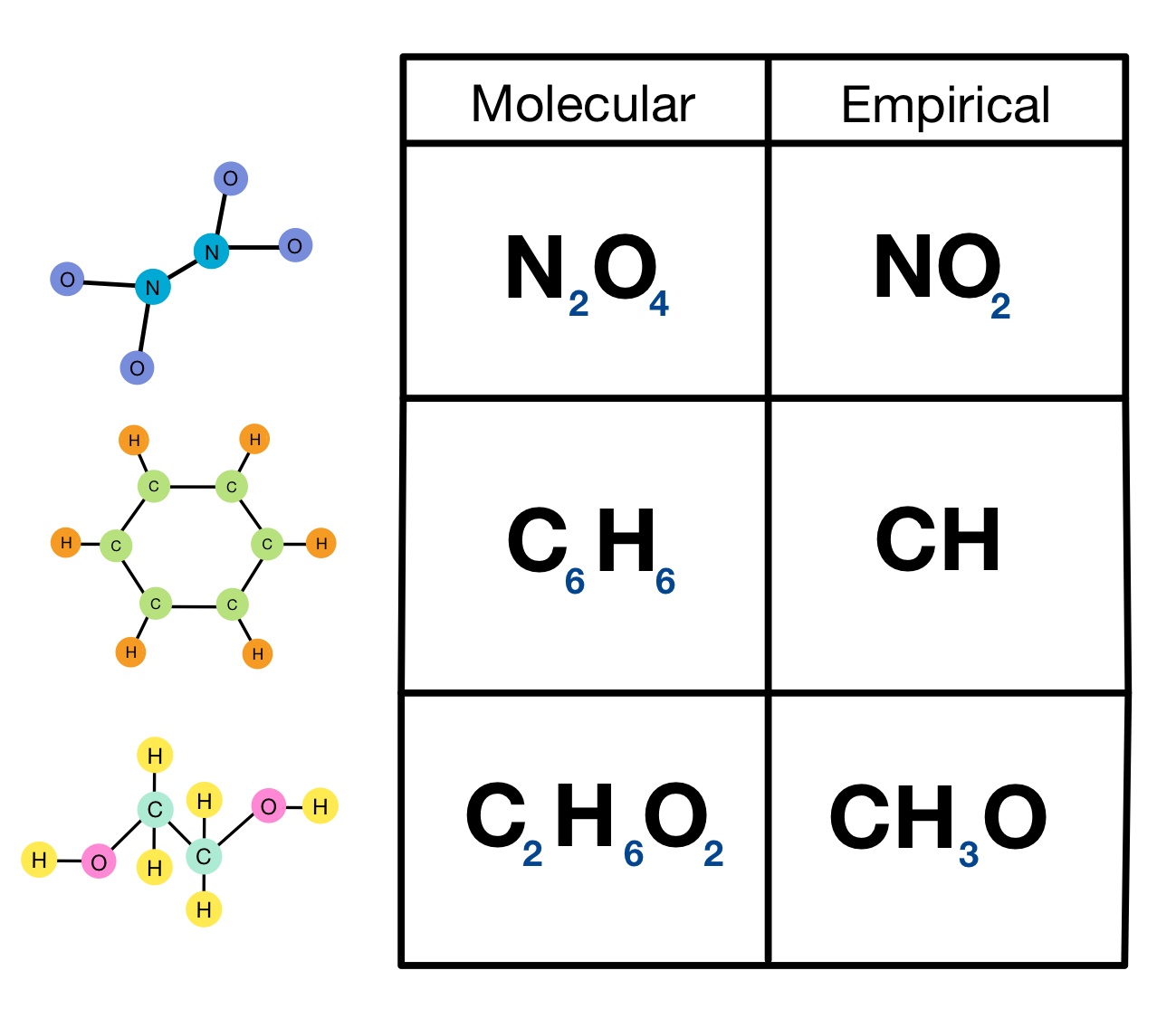
Empirical Formulas Overview Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/empirical_formula_examples.jpeg
Empirical and Molecular Formula Worksheet Name Write the empirical formula 1 Na 2 SO 4 2 C 6 H 12 O 6 3 C 4 H 10 4 KNO 2 5 H 2 O 2 Show all work for the following problems 6 Propene has an empirical formula of CH 2 What is its molecular formula if it has a molar mass of 42 09 g mol 7 Empirical and Molecular Formula Worksheet 1 An unknown hydrocarbon contains 20 hydrogen and 80 carbon Find the empirical formula of this hydrocarbon If its molar mass is 30g what is its molecular formula EF MF 2 An unknown base contains 54 1 calcium 43 2 oxygen and 2 7 hydrogen What is this base s empirical formula
Once you find the ratios you can find the empirical and the molecular formula 1 Vitamin C M 176 12 g mol is a compound of composed of carbon hydrogen and oxygen EMPIRICAL FORMULAS To determine the empirical formula of a compound 1 Determine the relative weights of the elements that make up the compound if they have not already been provided 2 Express these quantities in moles 3 Divide the number of moles by the minimum number of moles for each element 4 Create a ratio for the elements in the
More picture related to Molecular And Empirical Formula Worksheet
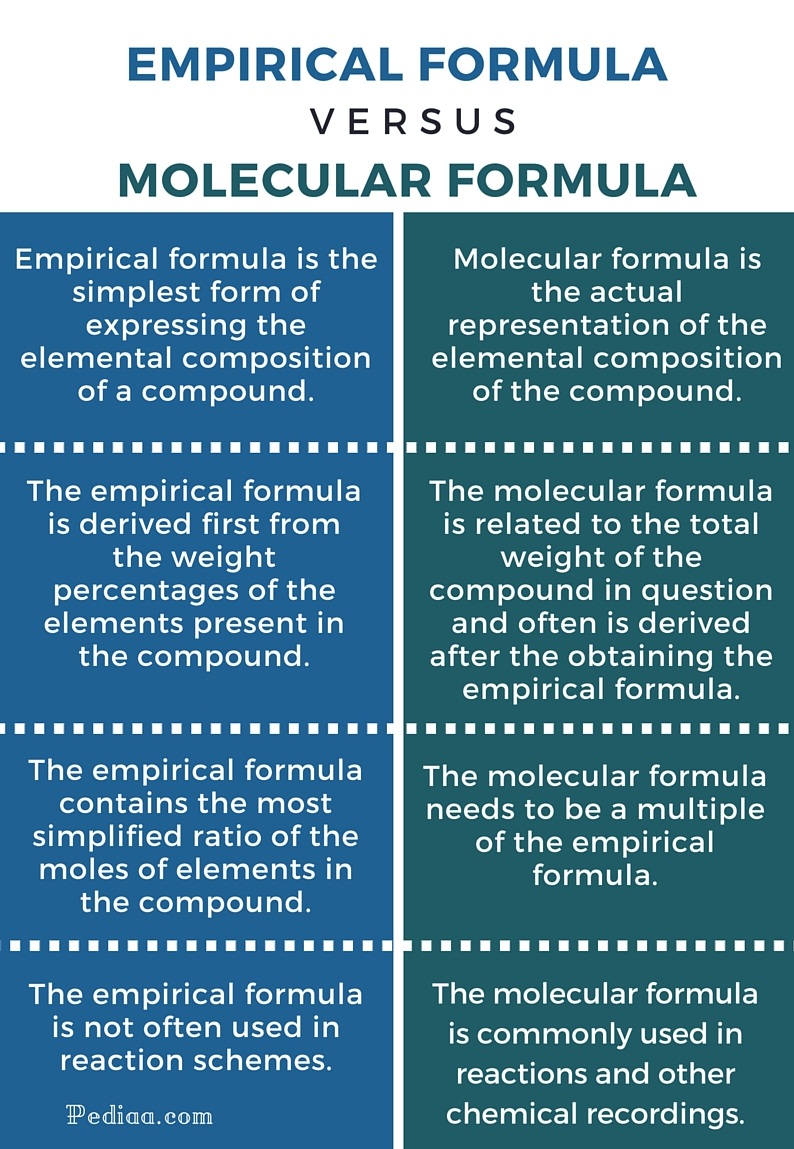
Difference Between Empirical And Molecular Formula
http://pediaa.com/wp-content/uploads/2015/12/Difference-Between-Empirical-and-Molecular-Formula-infographic.jpg

Empirical Molecular Formulas
https://s3.studylib.net/store/data/006806758_2-8cd1f4c9b04cf1a8ef2dbee3496cac07.png
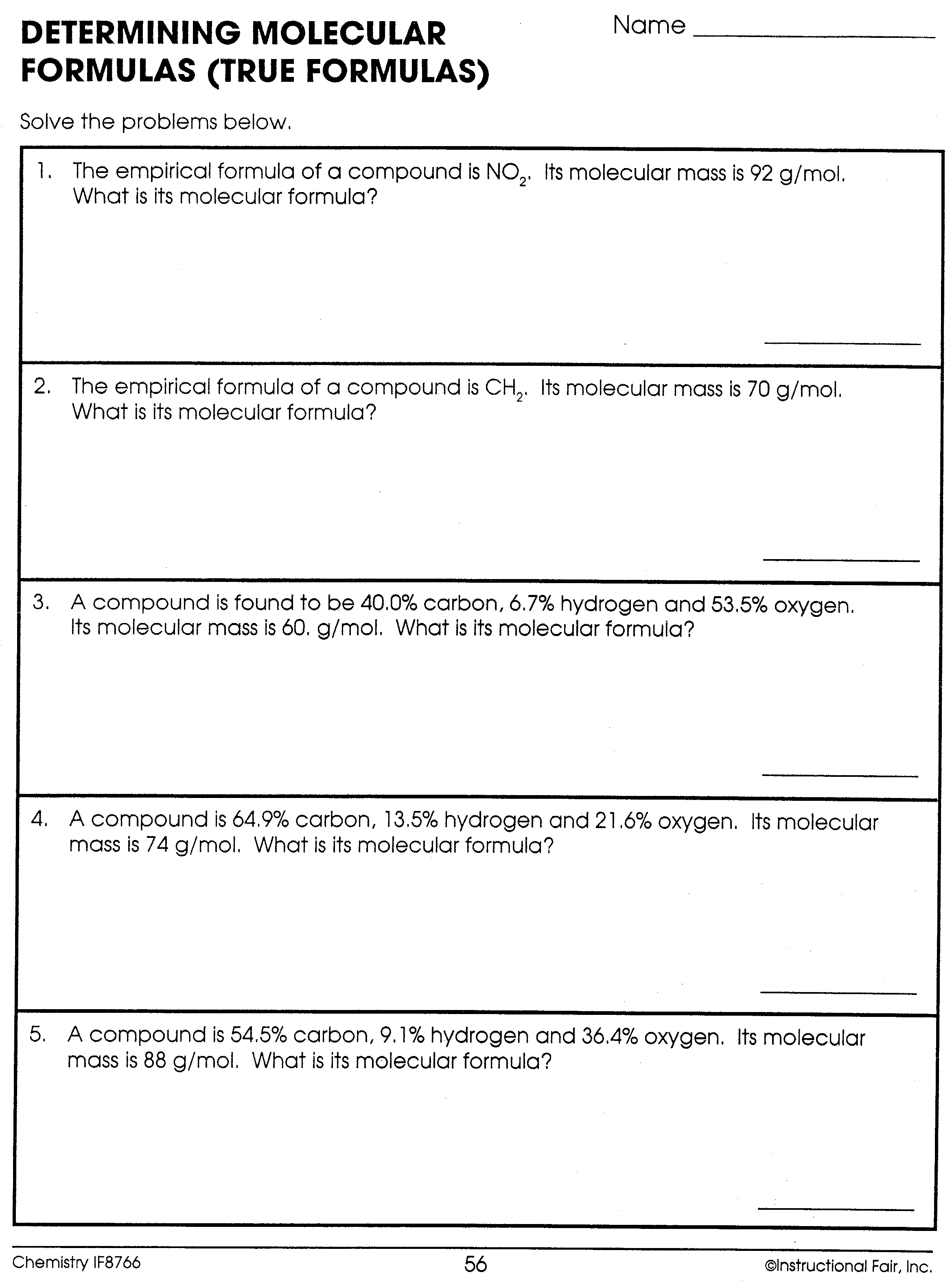
Daniel Davis January 2009 Archives
http://66.39.52.159/ddavis/CHif56.bmp
Empirical Formulas Worksheet Objectives be able to calculate empirical formulas Empirical formula Expresses the simplest ratios of atoms in a compound Written with the smallest whole number subscripts Molecular formula Expresses the actual number of atoms in a compound Empirical and Molecular Formula Worksheet Write the empirical formula for the following compounds 1 C 6 H 6 2 C 8 H 18 3 WO 2 4 C 2 H 6 O 2 5 X 39 Y 13 6 A compound with an empirical formula of C 2 OH 4 and a molar mass of 88 grams per mole What is the molecular formula of this compound 7 A compound with an empirical formula of C 4 H 4
[desc-10] [desc-11]

Science Density Calculations Worksheets
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/04/density-calculations-worksheet-answer-key.jpg

Empirical And Molecular Formula Worksheet Printable Pdf Download
https://data.formsbank.com/pdf_docs_html/88/884/88467/page_1_thumb_big.png
Molecular And Empirical Formula Worksheet - [desc-14]