Molecular And Empirical Formula Worksheet Pdf PDF 1 5 191 247 162 254 4 0 obj Linearized 1 L 89208 H 869 159 O 8 E 87060 N 2 T 88918 gt gt endobj 5 0 obj Type XRef Length 86 Filter FlateDecode
Empirical and Molecular Formulas Worksheet 1 What is the empirical formula for C8H18 2 What is the empirical formula for H2O 3 What is the empirical formula for C4H10 4 What is the empirical formula for C2H4O2 5 Is CO2 an empirical To calculate molecular formulas follow the steps outlined below Step 1 calculate empirical formula see above Step 2 divide the molecular formula mass given to you in the problem by the empirical
Molecular And Empirical Formula Worksheet Pdf

Molecular And Empirical Formula Worksheet Pdf
https://s3.studylib.net/store/data/007884987_2-0d8a2dde770adff75de1113cf0fd4dd0-768x994.png
Empirical Formula Worksheets Key
https://www.unmisravle.com/wp-content/uploads/2018/04/empirical_and_molecular_formula_worksheet_2.png

Chemistry Empirical Formula Worksheets
https://www.unmisravle.com/wp-content/uploads/2018/08/chemistry_empirical_formula_worksheet_answers_5299847_2.png
Empirical and Molecular Formula Worksheet Name Write the empirical formula 1 Na 2 SO 4 2 C 6 H 12 O 6 3 C 4 H 10 4 KNO 2 5 H 2 O 2 Show all work for the following problems 6 Propene has an empirical formula of CH 2 What is its molecular formula if it has a molar mass of 42 09 g mol 7 Empirical and Molecular Formula Worksheet 1 An unknown hydrocarbon contains 20 hydrogen and 80 carbon Find the empirical formula of this hydrocarbon If its molar mass is 30g what is its molecular formula EF MF 2 An unknown base contains 54 1 calcium 43 2 oxygen and 2 7 hydrogen What is this base s empirical formula
Write the empirical formula for the following compounds A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole What is the molecular formula of this compound Empirical Formula and Molecular Formula Which formula is more informative Learning Objectives 1 Distinguish between Empirical Formula and Molecular Formula 2 Determine a Molecular Formula given molecular mass and empirical formula for a compound 3 Students will be able to convert between empirical and molecular formulas thereby observing
More picture related to Molecular And Empirical Formula Worksheet Pdf

Empirical And Molecular Formula Teaching Chemistry Chemistry
https://i.pinimg.com/736x/63/32/6b/63326b51a2c04338fb561c01367aa5e8.jpg

Empirical Formula Worksheet 1 Chapter 8 Empirical Molecular Formulas
https://i.pinimg.com/originals/c7/60/43/c760437085fc9b3e75b8562e7ce45c0b.jpg

Empirical Molecular Formula Practice Worksheets
https://storage.googleapis.com/worksheetzone/image/63a52d6f44096e201a26cdc1/percent-composition-and-molecular-formula-worksheet-w1000-h1291-preview-4.png
Empirical Formula Example Problems 1 A compound is analyzed and found to contain 79 8 g C and 20 2 g H Determine the empirical formula 2 What is the empirical formula of a compound that is 25 9 g nitrogen and 74 1 g oxygen Determine the empirical and molecular formulas
EMPIRICAL FORMULAS To determine the empirical formula of a compound 1 Determine the relative weights of the elements that make up the compound if they have not already been provided 2 Express these quantities in moles 3 Divide the number of moles by the minimum number of moles for each element 4 Create a ratio for the elements in the Once you find the ratios you can find the empirical and the molecular formula 1 Vitamin C M 176 12 g mol is a compound of composed of carbon hydrogen and oxygen

Determining Empirical Formulas Worksheets
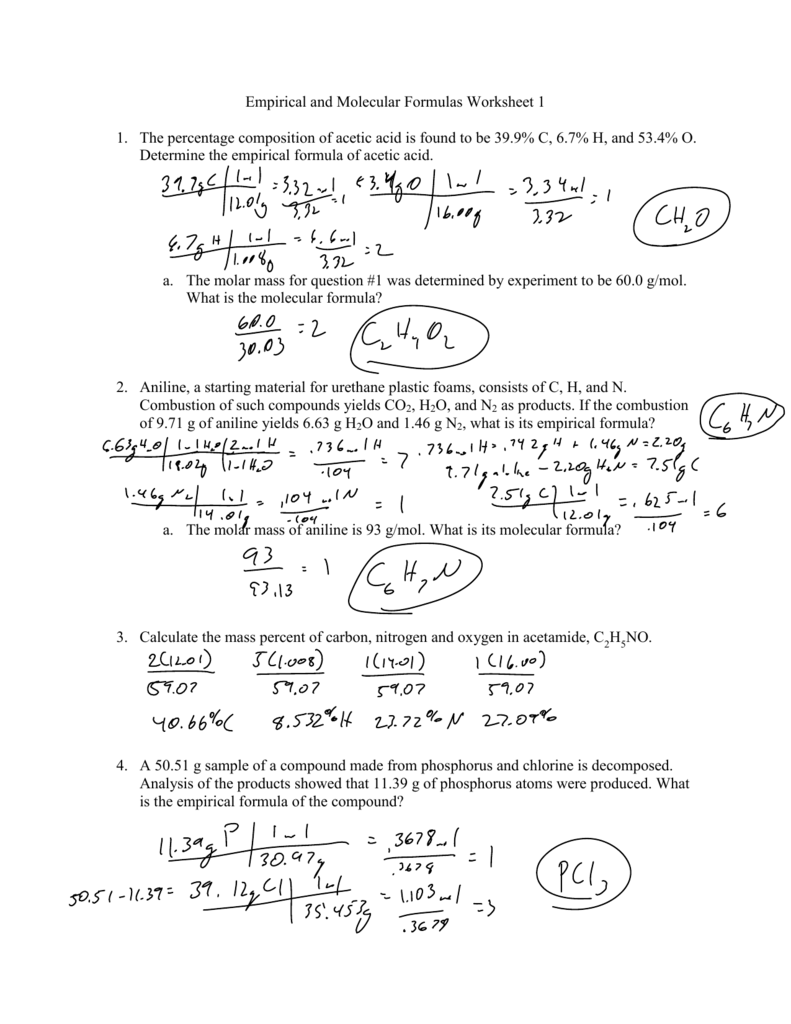
https://db-excel.com/wp-content/uploads/2019/09/empirical-and-molecular-formulas-worksheet-1-1-the-percentage-749x970.png

13 Best Images Of AP Chemistry Empirical Formula Worksheet Molecular
http://www.worksheeto.com/postpic/2015/12/molecular-and-empirical-formula-worksheet_690810.png
Molecular And Empirical Formula Worksheet Pdf - A compound has the empirical formula C3H6O and a molecular mass of 174 240 g mol What is the molecular formula of this compound Find the molar mass of the empirical formula