Mass To Mass Stoichiometry Worksheet Pdf With Answers How many moles of water are produced when 57 moles of nitrogen are made 3 Calculate the mass of aluminum oxide produced when 3 75 moles of aluminum burn in oxygen Answers 1A 30 mol Ag 1B 30 mol AgNO3 1C 20 mol H2O 1D 10 mol NO 2A 38 mol N2H4 2B 19 mol N2O4 2C 76 mol H2O
Step 1 Fill in Mr values and undertake a mass balance 2NaHCO3 Na2CO3 H2O CO2 Reaction Coefficients 2 1 1 1 Mr 84 168 106 18 44 Mass Balance 106 18 44 168 168 Mass No of moles Step 2 Fill in all the information you have been given in the question CO2 Reaction Coefficients Mr Mass Balance Mass No of moles CHM 130 Stoichiometry Worksheet The following flow chart may help you work stoichiometry problems Remember to pay careful 2 CO 2 g A Calculate the mass of ethanol produced if 500 0 grams of glucose reacts completely B Calculate the volume of carbon dioxide gas produced at STP if 100 0 grams of glucose reacts C If 17 5 moles of
Mass To Mass Stoichiometry Worksheet Pdf With Answers

Mass To Mass Stoichiometry Worksheet Pdf With Answers
https://s3.studylib.net/store/data/008548006_1-87998e5b1b015fdc34a74176a67dd4ab.png
Stoichiometry Worksheet Answers
https://imgv2-1-f.scribdassets.com/img/document/131383825/original/a396110907/1521550172?v=1
Stoichiometry Problem Set ANSWERS pdf
https://imgv2-2-f.scribdassets.com/img/document/392402324/original/c2bcc9e0dc/1597983252?v=1
Example 12 4 1 12 4 1 Mass Mass Stoichiometry Ammonium nitrate decomposes to dinitrogen monoxide and water according to the following equation NH4NO3 s N2O g 2H2O l NH 4 NO 3 s N 2 O g 2 H 2 O l In a certain experiment 45 7g 45 7 g of ammonium nitrate is decomposed Find the mass of each of the products formed Apply mass to mass stoichiometry Table salt or sodium chloride can be decomposed into sodium metal and chlorine gas by running electricity through molten NaCl A Downs cell produces sodium and chlorine from molten sodium chloride If 42 8 grams of Cl A 2 are produced by the above reaction what mass of NaCl reacted
No Question Answer 7 7 69 g of ammonium sulfate is added to a large volume of barium bromide solution to produce a precipitate of barium sulfate according to the equation NH 4 2 SO 4 s BaBr 2 aq BaSO 4 s 2NH 4 Br aq Calculate the mass of precipitate formed as a result of this reaction 8 What mass of phosphorus trichloride Moles of Fe 2 O 3 cancel leaving moles of SO 3 as the units This answer may then be converted to grams of SO 3 10 7 7 mol SO3 80 07gSO3 1 molSO3 862gSO3 The final answer is expressed to three significant figures Thus in a two step process we find that 862 g of SO 3 will react with 3 59 mol of Fe 2 O 3
More picture related to Mass To Mass Stoichiometry Worksheet Pdf With Answers
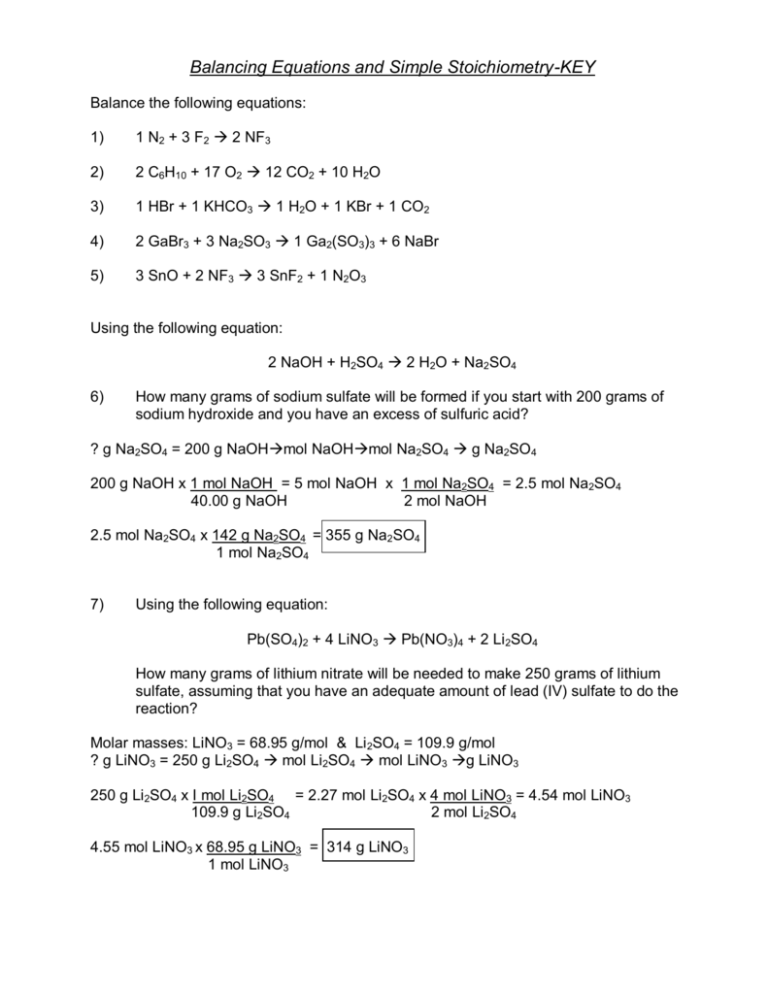
KEY Solutions For The Stoichiometry Practice Worksheet
https://s3.studylib.net/store/data/008711874_1-d12f29b6b62688faeae95fc3bd7c9543-768x994.png

FREE 9 Sample Stoichiometry Worksheet Templates In MS Word PDF
https://images.sampletemplates.com/wp-content/uploads/2016/11/17162629/Mixed-Stoichiometry-Worksheet.jpg

Mass Mass Stoichiometry
http://image.slidesharecdn.com/chemistrystoichiometry-130218190445-phpapp01/95/chemistry-stoichiometry-9-638.jpg?cb=1361214325
To do this start by dividing the smallest number of moles into each of the numbers of moles of elements i e set the smallest number to 1 This may yield integers or it may yield decimal results that correspond closely to rational fractions For example 1 25 2 75 1 2 5 11 1 25 2 75 1 2 5 11 This is a great quick worksheet to use as practice or review for mass to mass stoichiometry conversions It includes an answer key showing all of the work and units for each question Rated 5 out of 5 based on 1 reviews
Sample Problem Mass Mass Stoichiometry Ammonium nitrate decomposes to dinitrogen monoxide and water according to the following equation NH 4 NO 3 s N 2 O g 2 H 2 O l In a certain experiment 45 7 g of ammonium nitrate is decomposed Find the mass of each of the products formed Step 1 List the known quantities and plan the Worksheet 4 4 Solutions Mass mass stoichiometry Page 1 Pearson Education Australia a division of Pearson Australia Group Pty Ltd 2008 This page from the
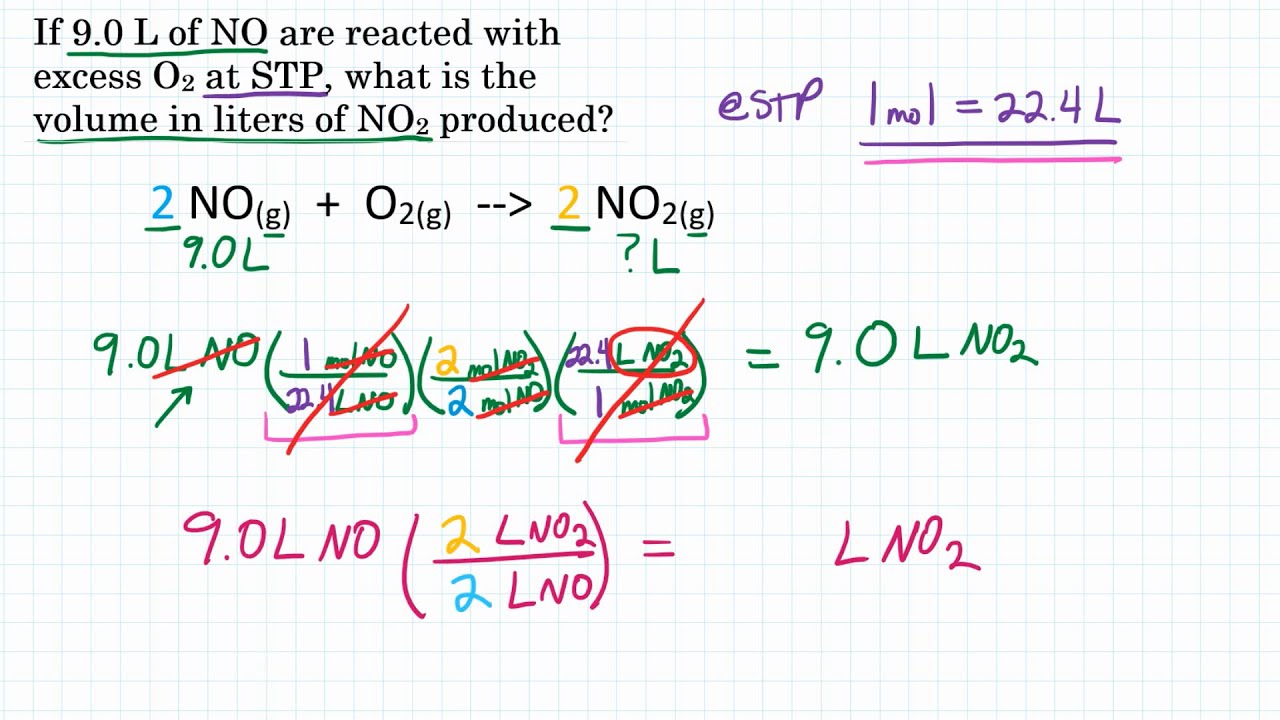
Ideal Gas Stoichiometry Volume To Volume Practice 1 YouTube
https://i.ytimg.com/vi/auF_HSWv0nE/maxresdefault.jpg

Mass To Mass Stoichiometry Worksheet Walkthrough YouTube
https://i.ytimg.com/vi/3SLbcaYBb8w/maxresdefault.jpg
Mass To Mass Stoichiometry Worksheet Pdf With Answers - Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry Balance the following equations 1 N Answer the following stoichiometry related questions 12 Write the balanced equation for the reaction of acetic acid with aluminum Using the equation from problem 12 determine the mass of aluminum acetate that can

