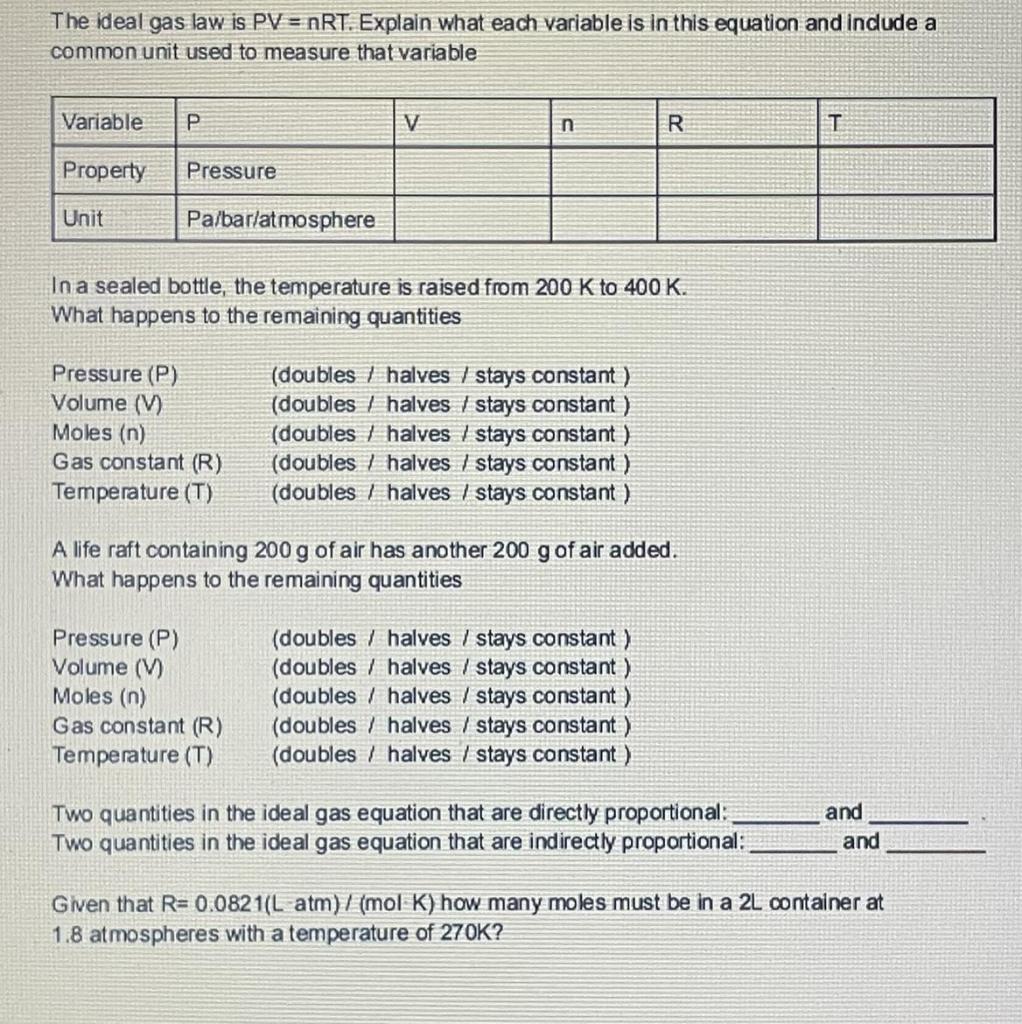
Ideal Gas Law Worksheet Pv Nrt Answers Ideal Gas Law Worksheet PV nRT Use the ideal gas law PerV nRT and the universal gas constant R 0 0821 L atm to solve the following problems K mol If pressure is needed in kPa then convert by multiplying by 101 3kPa 1atm to get R 8 31 kPa L K mole 1 If I have 4 moles of a gas at a pressure of 5 6 atm and a volume of 12
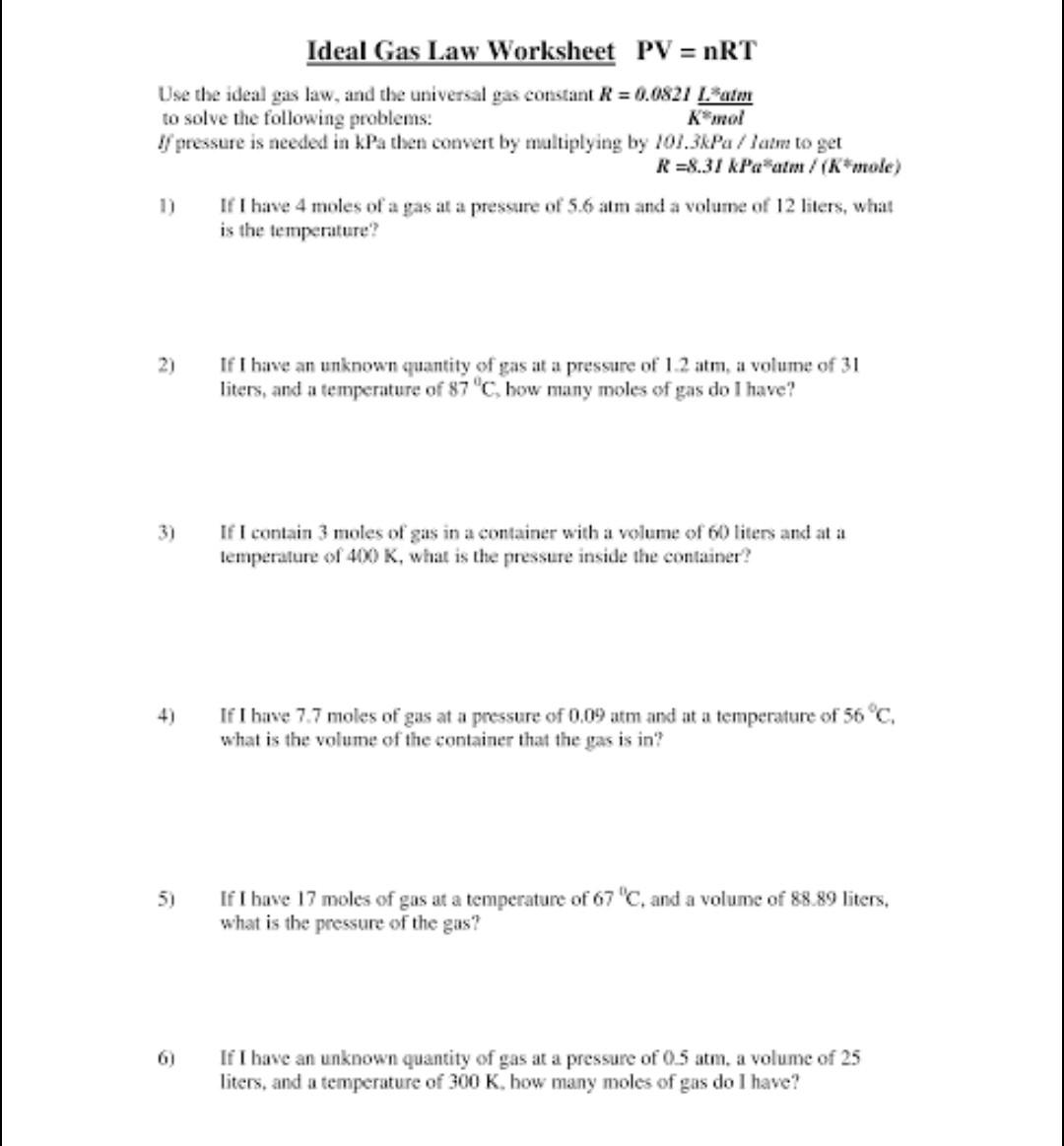
The Ideal and Combined Gas Laws PV nRT or P1V1 P2V2 Use your knowledge of the ideal and combined gas laws to solve the following problems If it involves moles or grams it must be PV nRT 1 If four moles of a gas at a pressure of 5 4 atmospheres have a volume of 120 liters what is the temperature The Ideal Gas Equation Before we look at the Ideal Gas Equation let us state the four gas variables and one constant for a better understanding The four gas variables are pressure P volume V number of mole of gas n and temperature T Lastly the constant in the equation shown below is R known as the the gas constant which will be discussed in depth further later
Ideal Gas Law Worksheet Pv Nrt Answers
Ideal Gas Law Worksheet Pv Nrt Answers
https://files.transtutors.com/book/qimg/55f8661b-b91b-4b84-a0af-3c4374ac195e.png

Ideal Gas Law Overview Calculations Expii
https://d20khd7ddkh5ls.cloudfront.net/ideal_gas_law_formula_2.jpeg
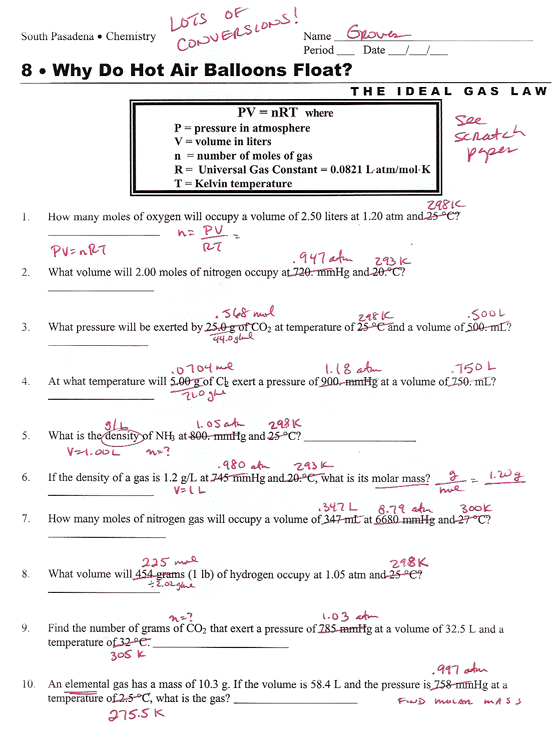
Ideal Gas Law Worksheet 14 4 Answer Key Greenus
http://www.chemmybear.com/groves/images/gcunit08_ideal1.gif
Expert Answer Solutions to the Ideal gas law practice worksheet The ideal gas law states that PV nRT where P is the pressure of a gas V is the volume of the gas n is the number of moles of gas present R is the ideal gas constant and T is the temperature of the gas in Kelvins Common mistakes Make sure you T in Kelvins rather than Worksheet CHEM 150 Ch 10 Ideal Gas Law 1 How many moles of gas air are in the lungs of an adult with a lung capacity of 3 9 L Use the equation PV nRT where R 0 082058 2 Calculate the volume occupied by 0 921 moles of nitrogen gas N 2 at a pressure of 1 38 atm and a temperature of 316 K 3 A sample of gas has a
Solutions to the Ideal gas law practice worksheet The ideal gas law states that PV nRT where P is the pressure of a gas V is the volume of the gas n is the number of moles of gas present R is the ideal gas constant and T is the temperature of the gas in Kelvins Common mistakes Students express T in degrees celsius rather than Kelvins The volume of a gas varies linearly with the number of moles V k A n k A is Avogadro s gas constant These are unified in the ideal gas law PV nRT R is the universal gas constant Critical thinking questions 1 Sketch on the graph below how the volume of a gas changes as the pressure is increased 2 Sketch on the graph below how
More picture related to Ideal Gas Law Worksheet Pv Nrt Answers

Ideal Gas Law Worksheet 2 Answer Ideal Gas Law Worksheet PV NRT Use
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/2fafe445515d2d847a7b1ce3a100c705/thumb_1200_1553.png
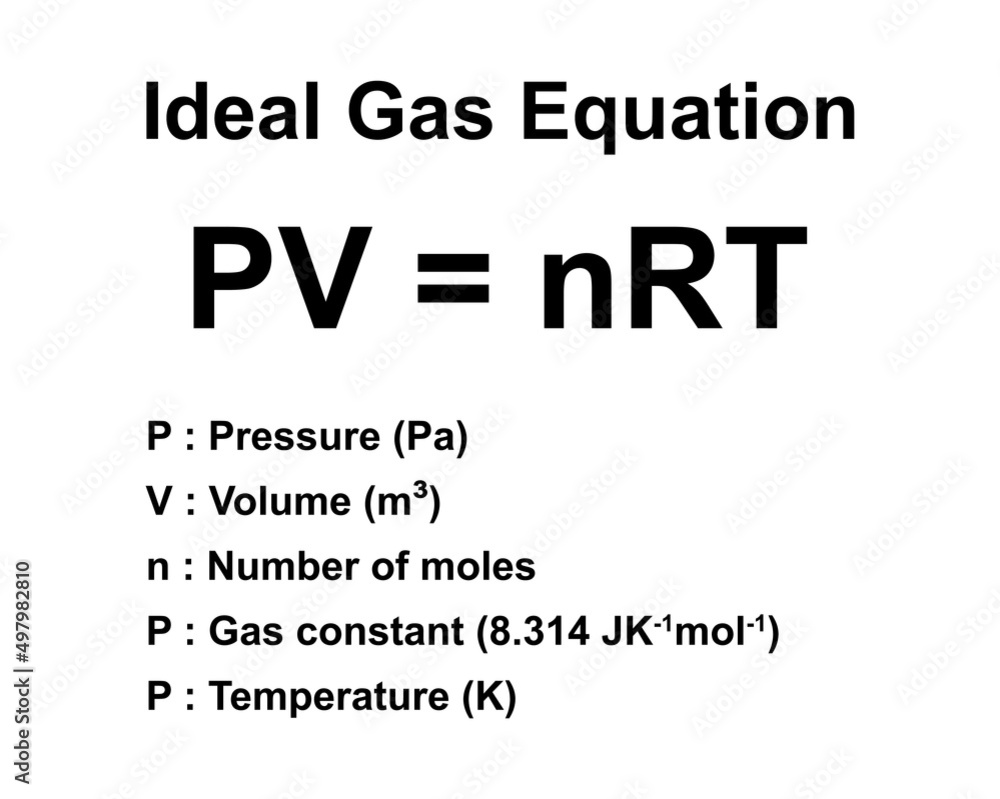
PV NRT Ideal Gas Law Brings Together Gas Properties The Most
https://as1.ftcdn.net/v2/jpg/04/97/98/28/1000_F_497982810_na4suI3Ptm8jOGE4DqGi1lzVxs7JMKUL.jpg
Solved The Ideal Gas Law Is PV NRT Explain What Each Chegg
https://media.cheggcdn.com/media/e70/e70ad28c-307d-4dc4-8b74-e784984d5087/php9QjZPj
Ideal Gas Law Worksheet PV nRT Use the ideal gas law PV nRT and the universal gas constant R 0 0821 L atm to solve the following problems K mol If pressure is needed in kPa then convert by multiplying by 101 3kPa 1atm to get R 8 31 L kPa K mole 1 If I have 4 moles of a gas at a pressure of 5 6 atm and a volume of 12 liters PROBLEM 7 3 1 10 7 3 1 10 Automobile air bags are inflated with nitrogen gas which is formed by the decomposition of solid sodium azide NaN 3 The other product is sodium metal Calculate the volume of nitrogen gas at 27 C and 756 torr formed by the decomposition of 125 g of sodium azide Answer
F H The ideal gas law PV nRT Worked example Using the ideal gas law to calculate number of moles Worked example Using the ideal gas law to calculate a change in volume Gas mixtures and partial pressures Express the answer using 3 significant figures

Ideal Gas Law Problems Worksheet Printable Word Searches
https://i2.wp.com/db-excel.com/wp-content/uploads/2019/09/the-ideal-and-combined-gas-laws-pv-nrt-or-p1v1-2-728x942.png
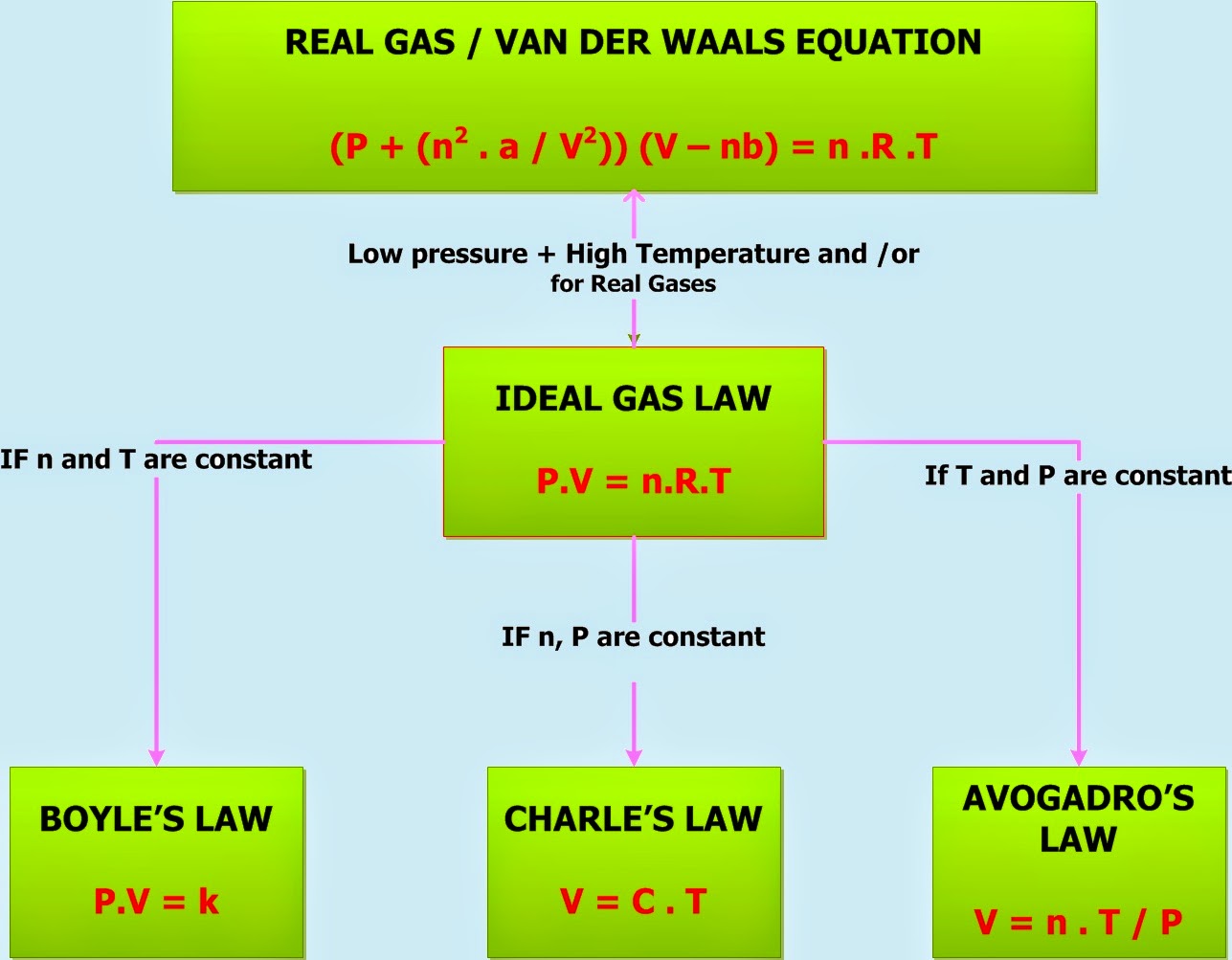
Gas Laws Ideal Gas Law Chemistry Net
http://2.bp.blogspot.com/-52PUTTjeHVw/VLu5_NLrJII/AAAAAAAABJQ/VMcC587XWLg/s1600/gas_laws1.jpg
Ideal Gas Law Worksheet Pv Nrt Answers - Worksheet CHEM 150 Ch 10 Ideal Gas Law 1 How many moles of gas air are in the lungs of an adult with a lung capacity of 3 9 L Use the equation PV nRT where R 0 082058 2 Calculate the volume occupied by 0 921 moles of nitrogen gas N 2 at a pressure of 1 38 atm and a temperature of 316 K 3 A sample of gas has a