Empirical And Molecular Formula Worksheet Pdf Empirical and Molecular Formula Worksheet Name Write the empirical formula 1 Na 2 SO 4 2 C 6 H 12 O 6 3 C 4 H 10 4 KNO 2 5 H 2 O 2 Show all work for the following problems 6 Propene has an empirical formula of CH 2 What is its molecular formula if it has a molar mass of 42 09 g mol 7
Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole The empirical formula of a compound is defined as the simplest whole number ratio of atoms of the elements in the compound The molecular formula of a compound is defined as the actual number of atoms of elements covalently bonded in a molecule and is a whole number ratio of the empirical formula
Empirical And Molecular Formula Worksheet Pdf
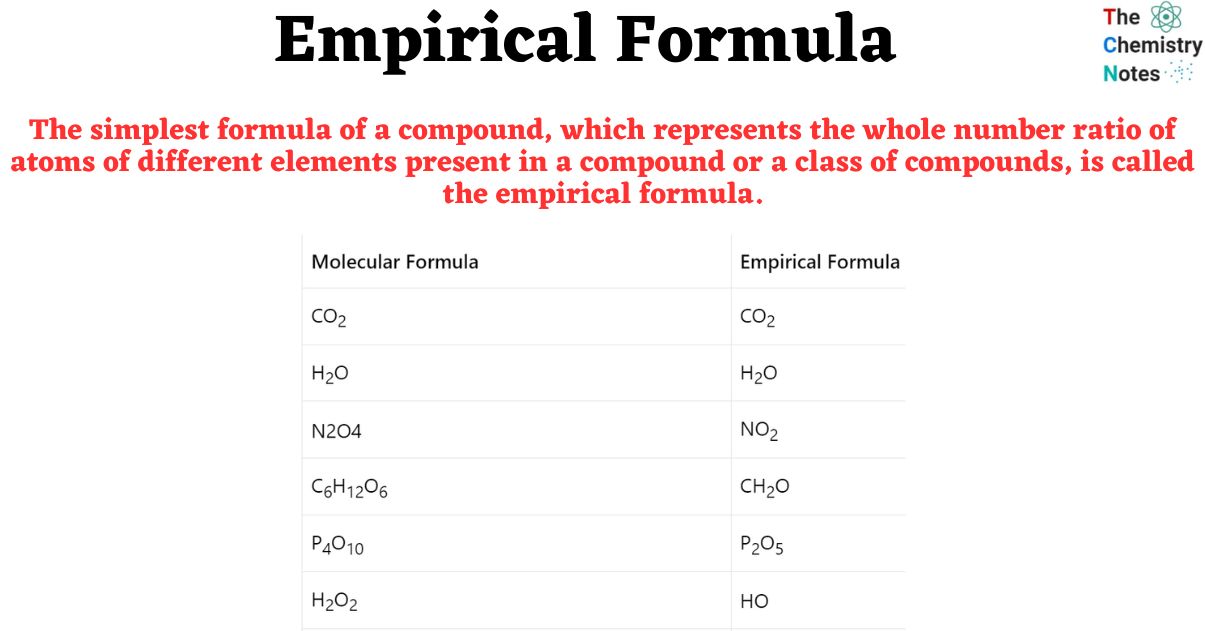
Empirical And Molecular Formula Worksheet Pdf
https://thechemistrynotes.com/wp-content/uploads/2023/08/The-simplest-formula-of-a-compound-which-represents-the-whole-number-ratio-of-atoms-of-different-elements-present-in-a-compound-or-a-class-of-compounds-is-called-the-empirical-formula.jpg

10 1 Distinguish Between Empirical Molecular And Structural Formulas
https://i.ytimg.com/vi/WkeOPe-Ia0U/maxresdefault.jpg

Determining Molecular Formula Worksheet
https://i2.wp.com/viziscience.com/wp-content/uploads/2017/03/empirical-formula2-1.png
Empirical and Molecular Formulas Worksheet 1 What is the empirical formula for C8H18 2 What is the empirical formula for H2O 3 What is the empirical formula for C4H10 4 What is the empirical formula for C2H4O2 5 Is CO2 an empirical Empirical and Molecular Formulas Worksheet 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O
Empirical and Molecular Formula Worksheet 1 An unknown hydrocarbon contains 20 hydrogen and 80 carbon Find the empirical formula of this hydrocarbon If its molar mass is 30g what is its molecular formula Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER Write the empirical formula for the following compounds 1 C 6H 6 2 C8H18 3 WO2 4 C2H6O2 5 X 39Y 13 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound
More picture related to Empirical And Molecular Formula Worksheet Pdf
Empirical Vs Molecular Formula
https://s3.studylib.net/store/data/009501907_1-29d062c715508933e624ab7981dd9c0f.png

Empirical molecular Formula Practice Worksheet Answer Key Printable
https://chessmuseum.org/wp-content/uploads/2019/10/empirical-and-molecular-formulas-worksheet-fresh-empirical-formula-worksheets-answer-key-of-empirical-and-molecular-formulas-worksheet.jpg

Empirical And Molecular Formula Practice Problems
https://s3.studylib.net/store/data/007884987_2-0d8a2dde770adff75de1113cf0fd4dd0.png
Empirical Formula Problems Set I 1 Determine the empirical formula of a compound consisting of a 55 3 K 14 6 P and 30 1 O K 3 PO 4 b 47 3 Cu and 52 7 Cl CuCl 2 c 52 14 C 13 13 H and 34 73 O C 2 H 6 O d 40 0 C 6 73 H and 53 3 O CH 2 O 2 In vanadium oxide the mole ratio is calculated to be 2 50 mol O 1 mol V EMPIRICAL FORMULAS To determine the empirical formula of a compound 1 Determine the relative weights of the elements that make up the compound if they have not already been provided 2 Express these quantities in moles 3 Divide the number of moles by the minimum number of moles for each element 4 Create a ratio for the elements in the
[desc-10] [desc-11]
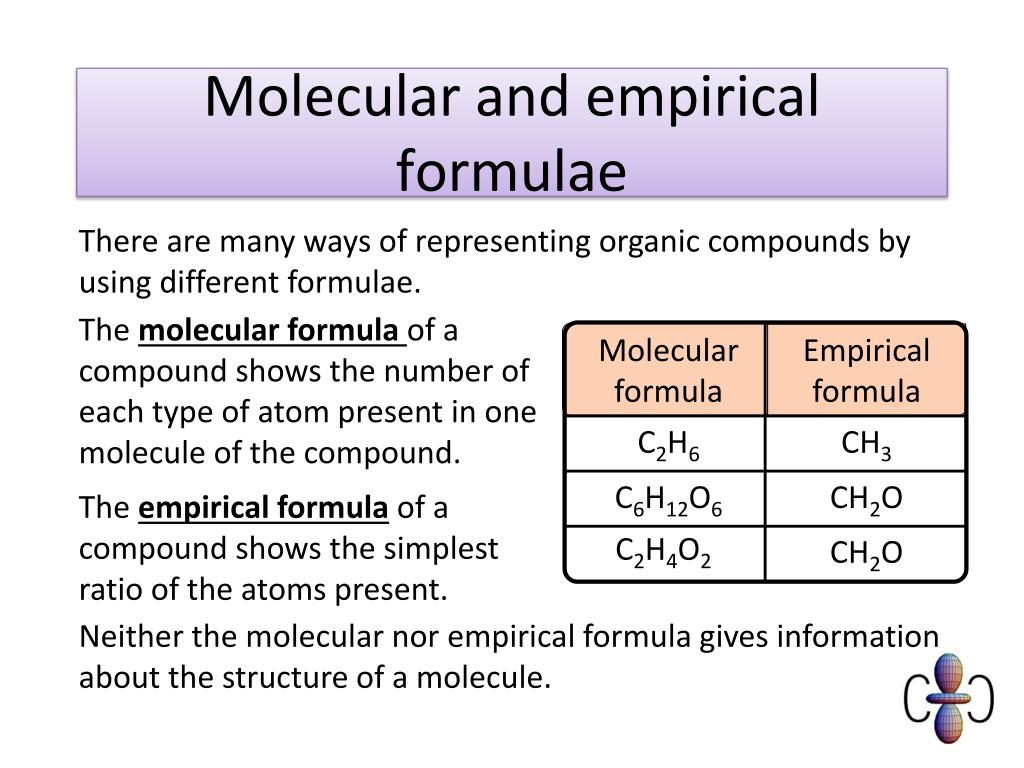
PPT Revision Guide Unit 2 PowerPoint Presentation Free Download
https://image1.slideserve.com/2019488/molecular-and-empirical-formulae-l.jpg

Empirical And Molecular Formula Worksheet PDF Document
https://cdn.vdocuments.mx/doc/1200x630/55cf9456550346f57ba15549/empirical-and-molecular-formula-worksheet-56196650141fb.jpg?t=1684478525
Empirical And Molecular Formula Worksheet Pdf - [desc-12]