Daltons Law Of Partial Pressures Worksheet Dalton s Law of Partial Pressures Worksheet 1 If I place 3 moles of N2 and 4 moles of O2 in a 35 L container at a temperature of 250 C what will the pressure of the resulting mixture of gases be 2 Two flasks are connected with a stopcock The first flask has a volume of 5 liters and contains nitrogen gas at a pressure of 0 75 atm
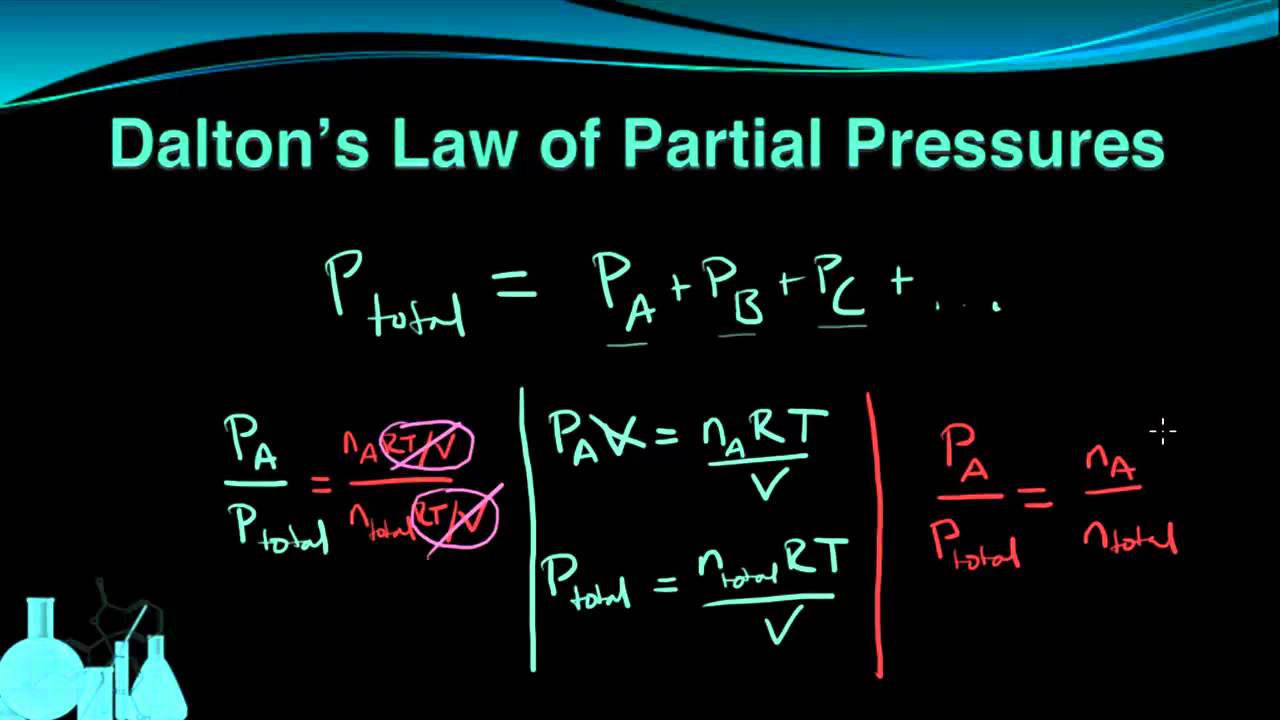
Dalton s law of partial pressures Dalton s law of partial pressures states that the total pressure of a mixture of gases is the sum of the partial pressures of its components P Total P gas 1 P gas 2 P gas 3 where the partial pressure of each gas is the pressure that the gas would exert if it was the only gas in the container The gases of three identical containers A B and C are under pressures of 1 44 atm 3 16 atm and 2 52 atm respectively These gases are then combined into Container D with a volume of 3 92 L so that the pressure in Container D is 4 38 atm Containers A B and C have the same volume Find that volume Answers 5 51 atm
Daltons Law Of Partial Pressures Worksheet

Daltons Law Of Partial Pressures Worksheet
https://storage.googleapis.com/worksheetzone/image/63a5292e44096e201a26b75b/daltons-law-of-partial-pressures-1681240443195-w1000-h1294-preview-0.png
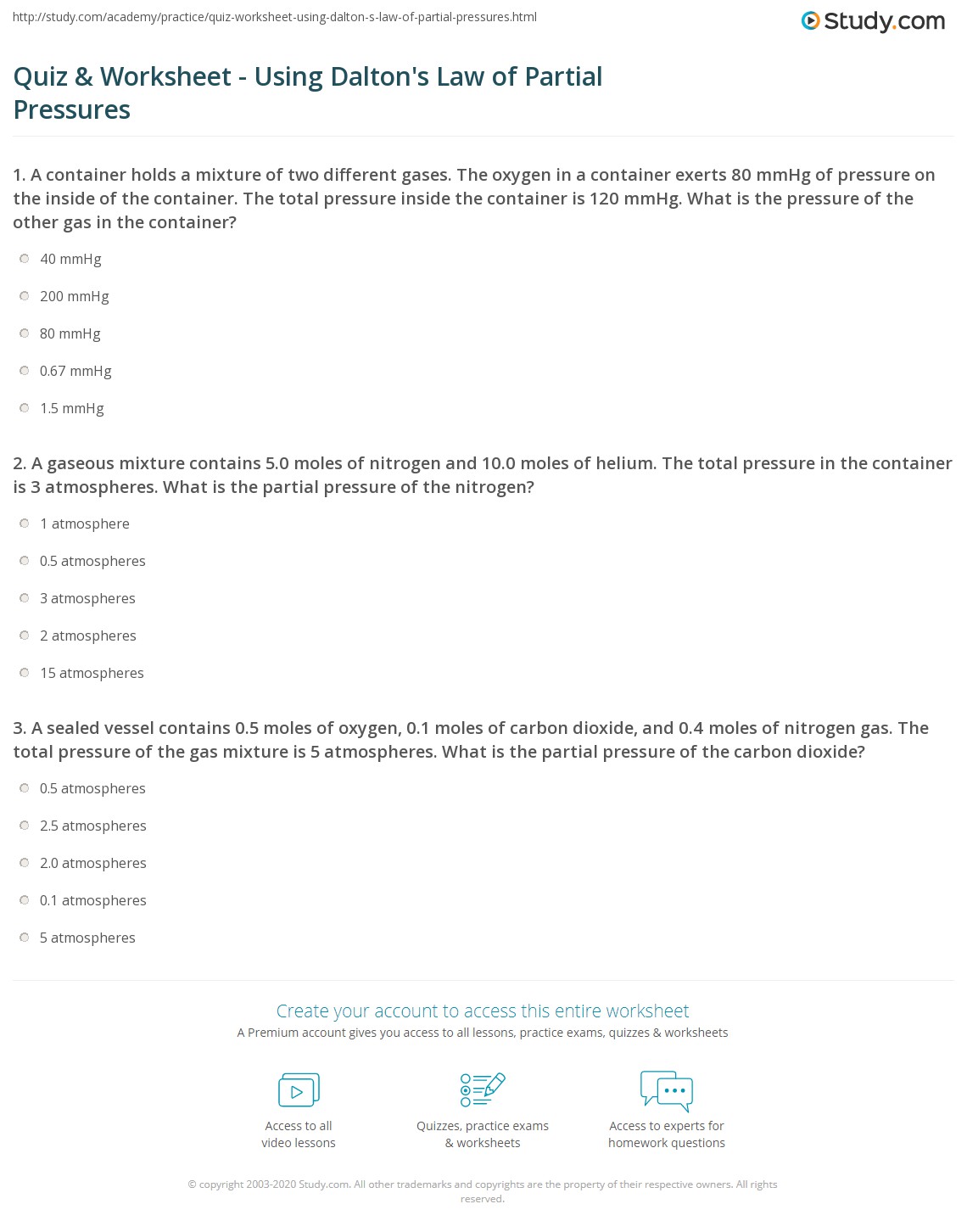
Quiz Worksheet Using Dalton s Law Of Partial Pressures Study
https://study.com/academy/practice/quiz-worksheet-using-dalton-s-law-of-partial-pressures.jpg

Dalton s Law Of Partial Pressure Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/12/Daltons-Law-of-Partial-Pressure.png
Created Date 2 28 2013 8 04 43 PM Worksheet C53 Dalton s Law of Partial Pressures Dalton s Law says that the sum of the individual pressures of all the gases that make up a mixture is equal to the total pressure or PT P1 P2 P3 The partial pressure of each gas is equal to the mole fraction of each gas times the total pressure
Dalton s law of partial pressure is an ideal gas law that states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas English scientist John Dalton observed the behavior of gases in 1801 and published the gas law in 1802 While Dalton s law of partial pressures describes ideal gases real Name period date Dalton s Law of Partial Pressure 1 A metal tank contains three gases oxygen helium and nitrogen
More picture related to Daltons Law Of Partial Pressures Worksheet

Dalton s Law Of Partial Pressure States Of Matter Physical
https://www.logiota.com/upload/img/898878718-10055452020.jpg
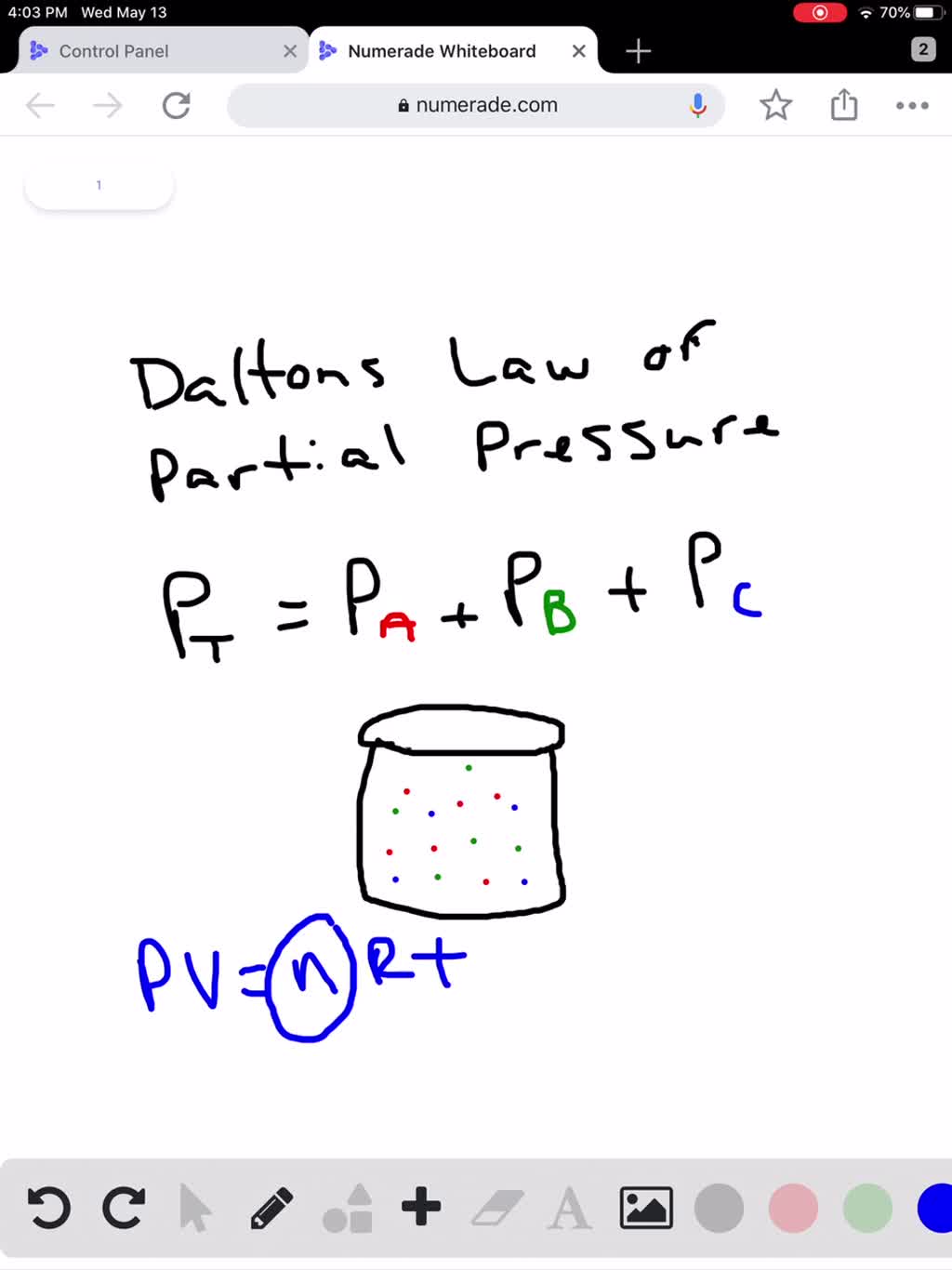
SOLVED How Does Dalton s Law Of Partial Pressures Help Us With Our
https://cdn.numerade.com/previews/59759064-52a3-429a-a18d-e94b649d49be_large.jpg
6 8 Partial Pressure Daltons Law Scuba Diving Blood
https://imgv2-2-f.scribdassets.com/img/document/342981053/original/fa4b0672bb/1585425936?v=1
Correct answer 0 042 mol of total gas At 304 K a 5 6 L container with H2 and N2 has a total pressure of 1 55 atm If there are 0 034 moles of H2 what is the partial pressure of N2 Step 1 Calculate the partial pressure of H2 V n RT 5 6 L 0 034 mol 0 08206 L atm mol K 304 K 0 15 atm Step 2 Calculate the partial pressure of N2 Dalton s Law of Partial Pressures Solve the following problems Show ALL work including equations and units Round all answers to the correct number of significant figures 1 A mixture of oxygen hydrogen and nitrogen gases exerts a total pressure of 278 kPa If the partial pressures of the oxygen and the hydrogen are 112 kPa and 101 kPa
Gases Liquids and Solids Dalton s Law Partial Pressure Simplified Practice Problems A 500 mL vessel contains a mixture of neon gas and chlorine gas Cl 2 The total pressure of the mixture at 28 0 C is 745 mmHg Calculate the partial pressure of chlorine if neon has a partial pressure of 148 mmHg Dalton s Law of Partial Pressures Worksheet 1 If I place 3 moles of N2 and 4 moles of O2 in a 35 L container at a temperature of 250 C what will the pressure of the resulting mixture of gases be 2 Two flasks are connected with a stopcock The first flask has a volume of 5 liters and contains nitrogen gas at a pressure of 0 75 atm
Dalton s Law Of Partial Pressure
https://i.ytimg.com/vi/gvXMg3J5j_Y/maxresdefault.jpg

30 Dalton s Law Of Partial Pressure Worksheet Worksheets Decoomo
https://i2.wp.com/image2.slideserve.com/4146302/dalton-s-law-of-partial-pressures-l.jpg
Daltons Law Of Partial Pressures Worksheet - Name period date Dalton s Law of Partial Pressure 1 A metal tank contains three gases oxygen helium and nitrogen
