Dalton S Law Of Partial Pressure Worksheet Doc Chemistry Dalton s Law of Partial Pressure Directions Solve each of the following problems Show your work including proper units to earn full credit 1 Container A with volume 1 23 dm3 contains a gas under 3 24 atm of pressure Container B with volume 0 93 dm3 contains a gas under 2 82 atm of
Dalton s Law of Partial Pressures Solve the following problems Show ALL work including equations and units Round all answers to the correct number of significant figures A mixture of oxygen hydrogen and nitrogen gases exerts a total pressure of 278 kPa Dalton s Law of Partial Pressures Worksheet 1 If I place 3 moles of N2 and 4 moles of O2 in a 35 L container at a temperature of 250 C what will the pressure of the resulting mixture of gases be 2 Two flasks are connected with a stopcock The first flask has a volume of 5 liters and contains nitrogen gas at a pressure of 0 75 atm
Dalton S Law Of Partial Pressure Worksheet Doc

Dalton S Law Of Partial Pressure Worksheet Doc
https://sbt.blob.core.windows.net/storyboards/85dadb25/dalton-s-law-of-partial-pressure.png?utc=132875125861300000

Dalton s Law Of Partial Pressure Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/12/Daltons-Law-of-Partial-Pressure.png
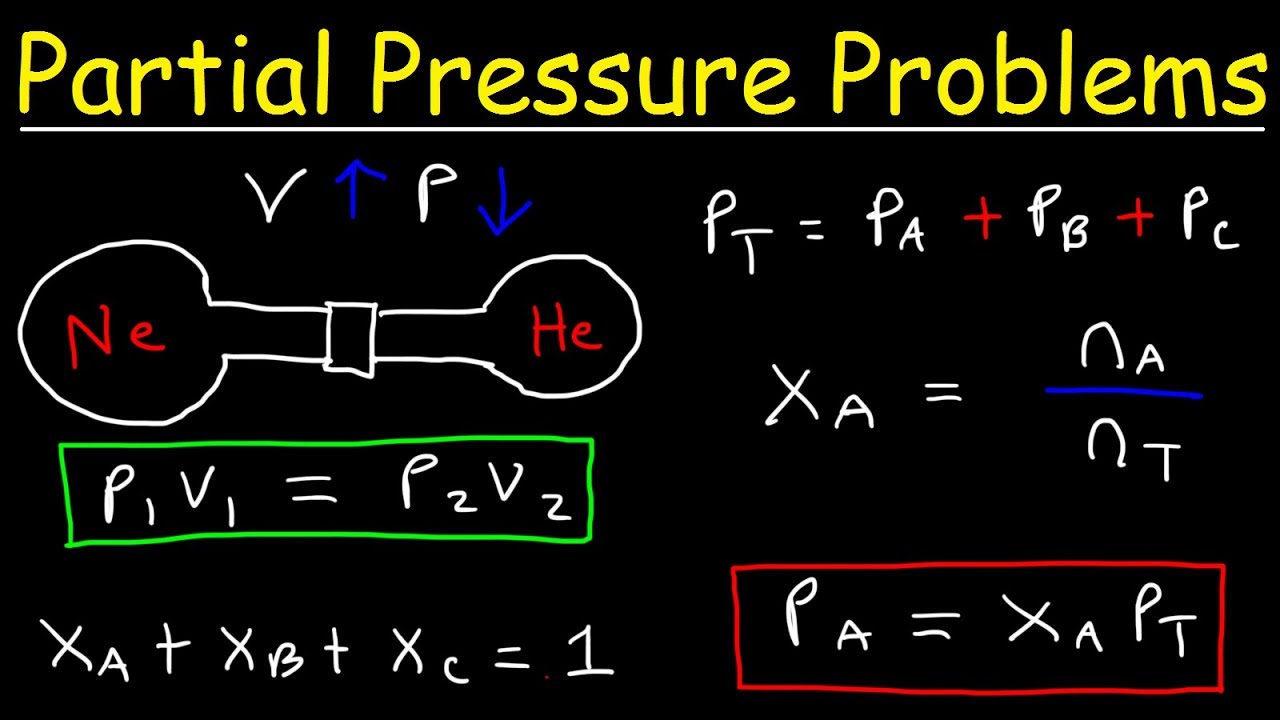
Dalton s Law Of Partial Pressure Problems Mole Fraction Chemistry Gas
https://i.ytimg.com/vi/J7YRwU7IV8Q/maxresdefault.jpg
According to Dalton s law of partial pressures the overall pressure exerted by a mixture of gasses is equal to the sum of the partial pressures exerted by each individual gas in the mixture The English chemist John Dalton stated this empirical relationship in 1801 Dalton s Law Of Partial Pressure Problems 1 The volume of hydrogen collected over water is 453 mL at 18 176 C and 780 mm Hg What is its volume dry at STP 2 A 423 mL sample of dry oxygen at STP is transferred to a container over water at 22 176 C
Practice problems on calculating partial and total pressures of gas mixtures using Dalton s Law Ideal for high school chemistry students Practice Dalton s Law with this worksheet Includes partial pressure calculations using ideal gas law and Boyle s law Answers included
More picture related to Dalton S Law Of Partial Pressure Worksheet Doc

Dalton s Law Of Partial Pressures Demonstration YouTube
https://i.ytimg.com/vi/oo4hSiYxff8/maxresdefault.jpg

Dalton s Law Of Partial Pressure Ideal Gas Equation Gases And
https://i.pinimg.com/originals/1c/fe/fe/1cfefec6c29a474e1db6e88b185264a3.jpg
/Dalton-s_law_of_partial_pressures-56a1338a3df78cf7726858f7.png)
What Is Dalton s Law Of Partial Pressures
https://fthmb.tqn.com/XPa0_hZfU0IgNvhXYrEKIlZAwEI=/3076x1172/filters:fill(auto,1)/Dalton-s_law_of_partial_pressures-56a1338a3df78cf7726858f7.png
Honors Chemistry Dalton s Law of Partial Pressure Directions Solve each of the following problems Show your work 1 A 250 ml sample of oxygen is collected over water at 25C and 760 0 torr pressure What is the pressure of the dry gas alone Vapor pressure of water at 25C is on chart above 2 The document provides 8 practice problems for applying Dalton s Law of Partial Pressures The problems involve calculating partial pressures and total pressures of gas mixtures in containers of varying volumes and numbers of moles of different gases
Dalton s Law states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases The document provides 8 practice problems for applying Dalton s Law of Partial Pressures Chemistry Dalton s Law of Partial Pressure Directions Solve each of the following problems Show your work including proper units to earn full credit 1 Container A with volume 1 23 dm3 contains a gas under 3 24 atm of pressure Container B with volume 0 93 dm3 contains a gas under 2 82 atm of pressure

Dalton s Law Of Partial Pressure States Of Matter Physical
https://www.logiota.com/upload/img/898878718-10055452020.jpg

Chapter 11 17 PROBLEM Dalton s Law Of Partial Pressures YouTube
https://i.ytimg.com/vi/QBZcgwSHBew/maxresdefault.jpg
Dalton S Law Of Partial Pressure Worksheet Doc - Practice Dalton s Law with this worksheet Includes partial pressure calculations using ideal gas law and Boyle s law Answers included