Chemistry Empirical Formula Worksheet Answers Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in the problems are grams Step 1 convert to moles Step 2 divide each by the lowest number of moles Step 3 only if necessary multiply all by the same factor in order to obtain whole numbers
Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula
Chemistry Empirical Formula Worksheet Answers
Chemistry Empirical Formula Worksheet Answers
https://d20khd7ddkh5ls.cloudfront.net/empirical_formula_examples.jpeg
Solved Empirical Molecular Formula Practice Worksheet Chegg
https://media.cheggcdn.com/study/06d/06d3e824-b032-432d-b735-42d1c4017b3a/image

Chemical Reactions Worksheet Answers
https://images-na.ssl-images-amazon.com/images/I/81ixpcdJRVL.jpg
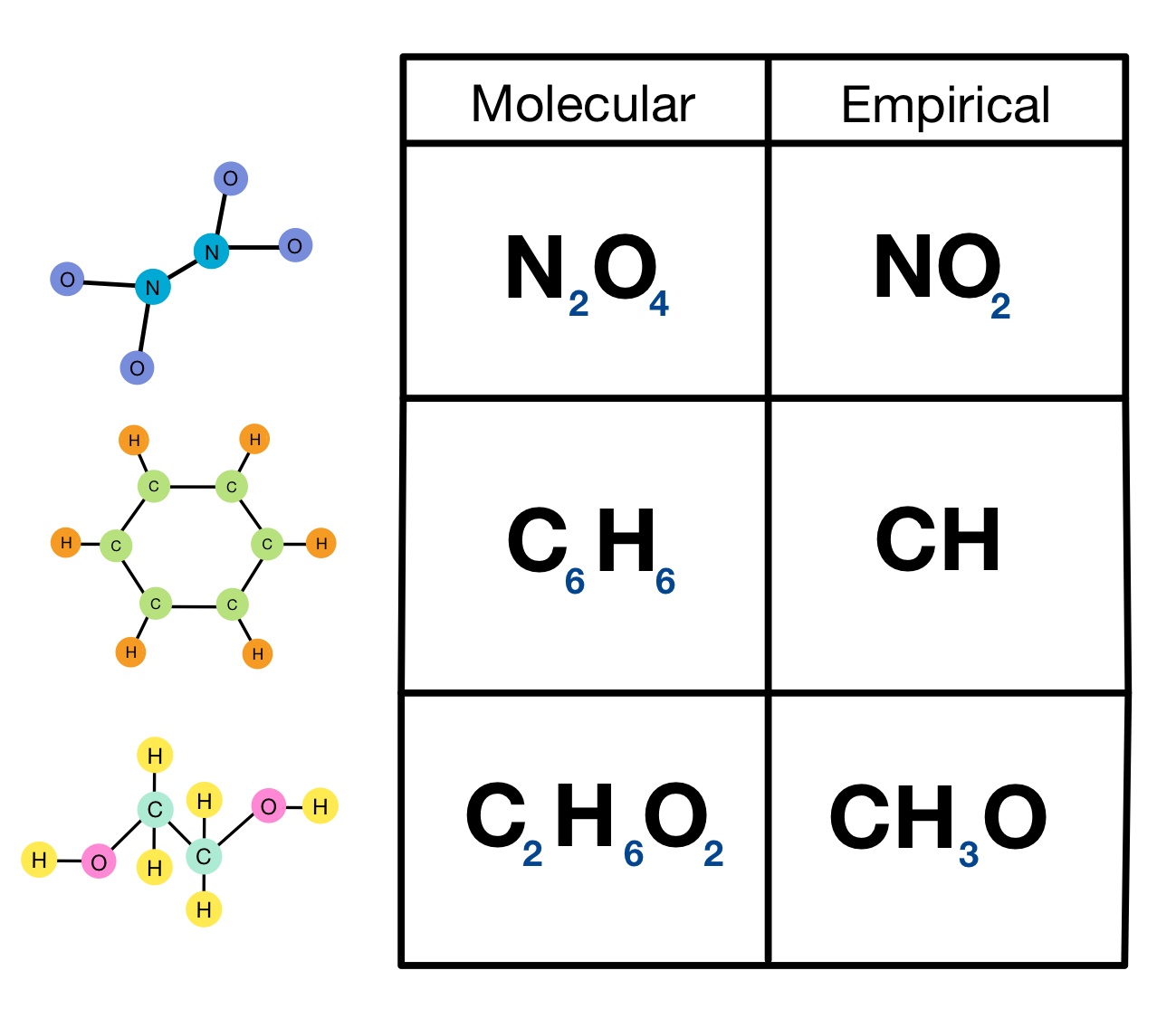
Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound 7 A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole For each formula below give the empirical formula Sometimes the formula given is the same as the empirical sometimes it is different a C 3 H 6 O 3 b N 2 O 4 c Mg 3 N 2 d C 7 H 14 O 2 e P 2 O 5 f C 4 H 8 N 2 Answer a and b
Show your work for the calculation of empirical formula here Excessive physical activity lactic acid molecular mass 90 08 g per mole forms in muscle tissues and is responsible for muscle soreness Elemental analysis shows that this compound has 40 0 carbon 6 71 hydrogen and 53 3 oxygen Determine the empirical formula of lactic acid Directions Find the empirical formula and name for each of the following A compound is 24 7 Calcium 1 2 Hydrogen 14 8 Carbon and 59 3 Oxygen Write the empirical formula and name the compound A compound is 21 20 Nitrogen 6 06 Hydrogen 24 30 Sulfur and 48 45 Oxygen Write the empirical formula and name the compound
More picture related to Chemistry Empirical Formula Worksheet Answers

Class 11 Chemistry Worksheet On Chapter 1 Some Basic Concepts Of
https://cdn1.byjus.com/wp-content/uploads/2022/09/Class-11-Chemistry-Worksheet-Chapter-1-Some-Basic-Concepts-of-Chemistry-Questions-Set-2.docx-3.jpg
From The Molecular Formula To The Empirical Formula YouTube
https://i.ytimg.com/vi/0Ikv_o7gcAE/maxresdefault.jpg
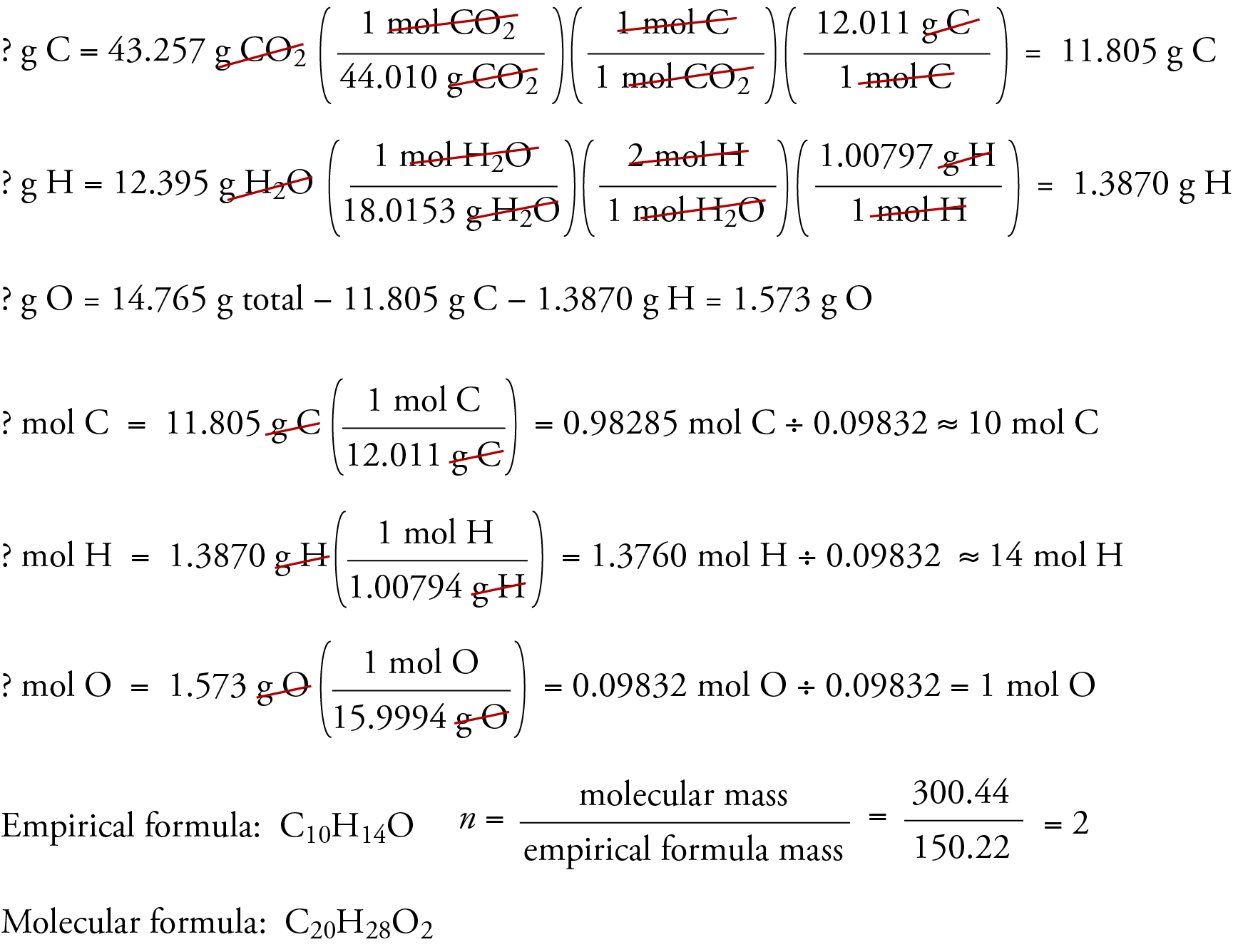
How To Find Molecular Formula
https://preparatorychemistry.com/images/molecular_formula_dianabol_CS.png
Empirical formula worksheet To print or download this file click the link below Empirical Formula Worksheet pdf PDF document 1 83 MB 1921394 bytes 2 Multiply all the subscripts in the empirical formula by the answer to the previous step Example 2 If the compound from Example 1 had a molecular weight of 306 g what would the molecular formula be Solution The empirical formula was A 2 O 3 The empirical formula weight is 2 27 0 g 3 16 0 g 102 g
OK first some corrections That was 73 by mass not 73 Hg and 27 by mass not 27 Cl But more importantly you have mistaken the number of moles a measure of the number of atoms of Hg Cl for their atomic weights a measure of the average weight of a collection of atoms of that element Number of moles Your answers should be whole numbers and are used as the subscripts in the chemical formula Step 4 Use the mole ratio to write the empirical formula Example Find the empirical formula for the compound that contains 42 05 g of nitrogen and 95 95 g of oxygen Step 1 Skip you were given grams

Empirical And Molecular Formula Practice By Teach Simple
https://teachsimplecom.s3.us-east-2.amazonaws.com/images/empirical-and-molecular-formula-practice/image-1653688677256-2.jpg

Some Basic Concepts Of Chemistry Class 11 Mole Concept L 2 NEET
https://i.ytimg.com/vi/nxBFsEO3lc4/maxresdefault.jpg
Chemistry Empirical Formula Worksheet Answers - Worksheet Molar Mass Answers 28 of the above worksheet problems in an HTML file Handwritten solutions Some Bonus Empirical Formula Problems Determine empirical formula from percent composition data 10 15 Determine empirical formula from mass data 10 10 Determine identity of an element from a binary formula and a percent
