Chemistry Empirical Formula Practice Answer Key Jul 29 2024 nbsp 0183 32 The empirical formula of a compound represents the simplest whole number ratio between the elements that make up the compound This practice test includes empirical formula questions that deal with finding empirical formulas of chemical compounds
E mp i r i c al M ol e c u l ar F or mu l a P r ac ti c e Wor k s h e e t te Score Empirical Molecular Formula Practice WorksheetDirections Find th empirical AND molecular formulas give 1 26 4 Carbon 3 3 Hydrogen 70 3 Oxygen Empirical Formula 2 81 8 grams Carbon Determine the empirical formulas for compounds with the following percent compositions a 15 8 carbon and 84 2 sulfur b 40 0 carbon 6 7 hydrogen and 53 3 oxygen Answer a Answer b Click here to see a video of the solution
Chemistry Empirical Formula Practice Answer Key

Chemistry Empirical Formula Practice Answer Key
https://db-excel.com/wp-content/uploads/2019/09/empirical-and-molecular-formulas-worksheet-1-1-the-percentage-749x970.png

Empirical Formula Worksheet 1
https://i.pinimg.com/originals/a7/b4/50/a7b4503e28fb0ae680f1bf867069ef17.png

Empirical And Molecular Formula Worksheets Answers
https://www.unmisravle.com/wp-content/uploads/2018/02/empirical_formula_worksheet_answers_free_worksheets_library_1.jpg
May 28 2020 nbsp 0183 32 Determine the empirical formulas for compounds with the following percent compositions a 15 8 carbon and 84 2 sulfur b 40 0 carbon 6 7 hydrogen and 53 3 oxygen Answer a CS 2 Answer b CH 2 O Click here to see a video of the solution Answer key Problem 1 A 15 67 g sample of a hydrate of magnesium carbonate was heated without decomposing the carbonate to drive off the water The mass was reduced to 7 58 g What is the formula of the hydrate Solution 1 Determine mass of water driven off 15 67 minus 7 58 8 09 g of water
Download Study notes Empirical and Molecular Formula Worksheet ANSWER KEY Brussels School of International Studies A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this What is the empirical formula of a compound that is 13 5 calcium 10 8 oxygen and 0 675 hydrogen by mass For full credit you must not only have the correct answer but show your step by step Q amp A
More picture related to Chemistry Empirical Formula Practice Answer Key

Empirical And Molecular Formula Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/empirical-and-molecular-formula-worksheet.png

Empirical Formula Worksheets 1 Answers
https://www.housview.com/wp-content/uploads/2018/03/empirical_formula_worksheet_1.jpg

Empirical And Molecular Formula Practice Solutions Docsity
https://static.docsity.com/documents_first_pages/2021/04/20/f27b8372eab457d749d70fed0e1dc877.png?v=1646976324
Identify the compound that has the same molecular formula and empirical formula Empirical Formulas Worksheet 1 Directions Find the empirical formula and name for each of the following 1 A compound is 24 7 Calcium 1 2 Hydrogen 14 8 Carbon and 59 3 Oxygen Write the empirical formula and name the compound 2 A compound is 21 20 Nitrogen 6 06 Hydrogen 24 30 Sulfur and 48 45 Oxygen
Past paper questions for the Empirical and Molecular Formula topic of A Level AQA Chemistry Objectives be able to calculate empirical and molecular formulas Empirical Formula 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O 3 What is empirical formula if compound
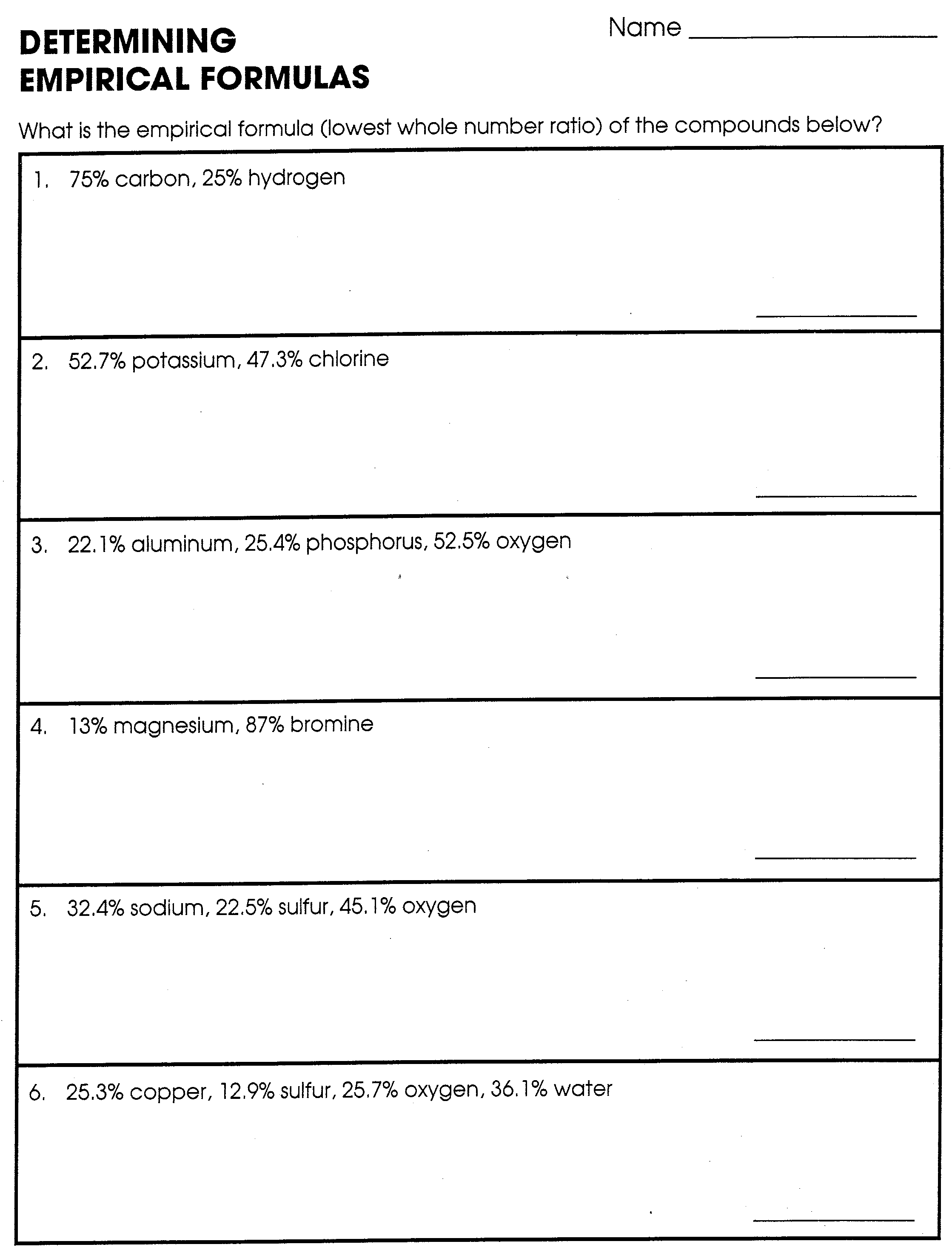
Molecular And Empirical Formula Worksheets
http://66.39.52.159/ddavis/CHif55.bmp

Empirical And Molecular Formula Worksheet Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/empirical-and-molecular-formulas-worksheet-1-1-the-percentage.png
Chemistry Empirical Formula Practice Answer Key - What is the empirical formula of a compound that is 13 5 calcium 10 8 oxygen and 0 675 hydrogen by mass For full credit you must not only have the correct answer but show your step by step Q amp A