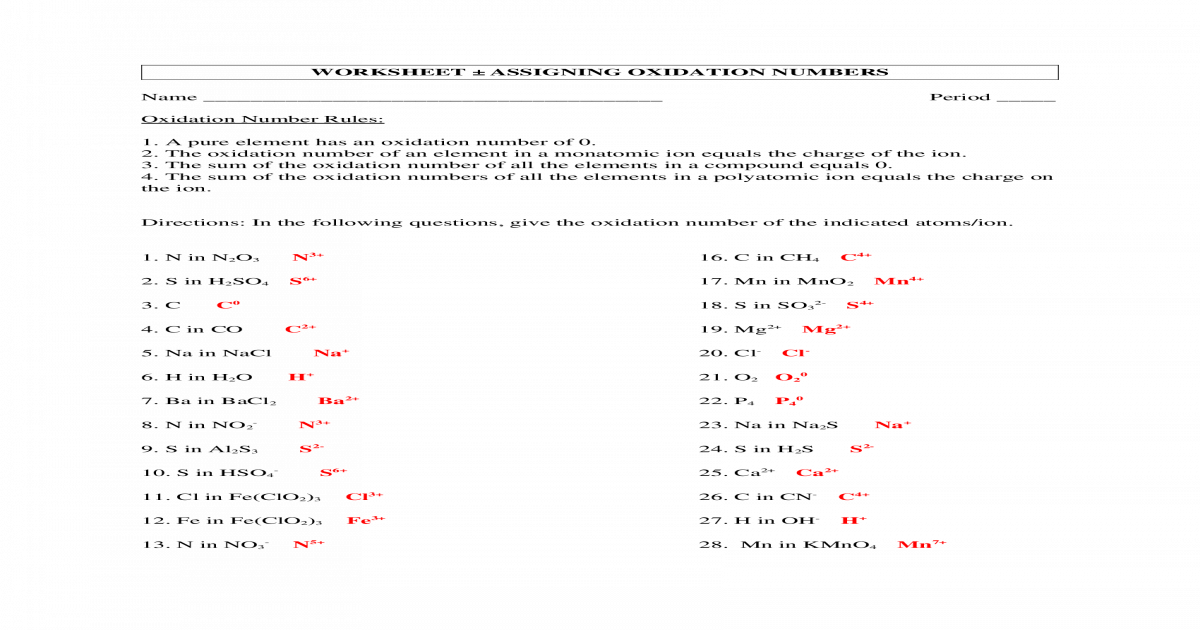
Assigning Oxidation Numbers Worksheet 1 Worksheet 1 Rules for Assigning Oxidation Numbers 1 The oxidation number of any uncombined element is 0 2 The oxidation number of a monatomic ion equals the charge on the ion 3 The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion
In the chlorate ion ClO 3 ClO 3 the oxidation number of Cl Cl is 5 5 and the oxidation number of O O is 2 2 In a neutral atom or molecule the sum of the oxidation numbers must be 0 In a polyatomic ion the sum of the oxidation numbers of all the atoms in the ion must be equal to the charge on the ion Example 22 6 1 22 The oxidation state of an atom is represented by a positive or negative number called its oxidation number Chemists have developed rules used to assign oxidation numbers The following rules will help you determine the oxidation state of an atom or ion A free atom has an oxidation number of zero It is not sharing gaining or losing electrons
Assigning Oxidation Numbers Worksheet 1
Assigning Oxidation Numbers Worksheet 1
https://img.pdfslide.net/img/1200x630/reader021/image/20170807/577c79841a28abe05492f726.png?t=1627368138

The Periodic Table Of Oxidation States Compound Interest
https://i0.wp.com/www.compoundchem.com/wp-content/uploads/2015/11/The-Periodic-Table-Of-Oxidation-States-2016.png
/113718222-56a12ee35f9b58b7d0bcd9dc.jpg)
Rules For Assigning Oxidation Numbers
https://fthmb.tqn.com/9CeXwb7Y-y9nc0OiJg7-T43nMhU=/4050x4268/filters:fill(auto,1)/113718222-56a12ee35f9b58b7d0bcd9dc.jpg
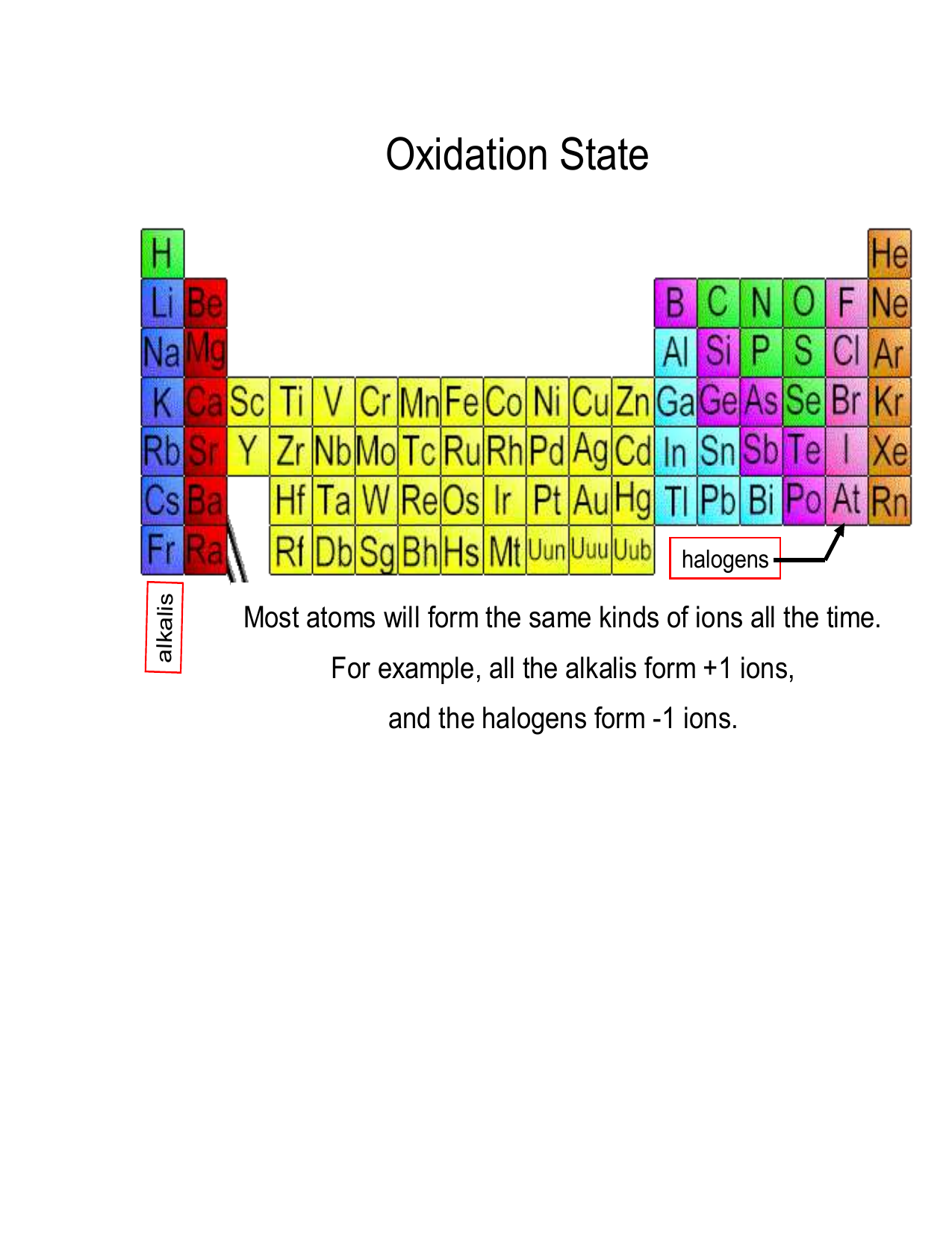
Student worksheet 30SW Oxidation numbers Page 5 of 6 Part 3 Before you start this activity you need to be proficient at assigning oxidation numbers to elements in compounds using the oxidation number rules 1 Assign oxidation numbers to the elements in the following species using the oxidation number rules a H 2 O 2 b S 2 O 3 2 c CrO 8 e g all Group 1 ions are 1 all group 2 ions are 2 all the following ions have oxidation numbers given by their charges Fe 2 Al 3 S 2 N 3 Fluorine is always 1 in its compounds Halogens are usually 1 except when a central atom or when combined with a more electronegative element e g assign I as 1 in NI 3 but 3 in ICl 3
Rules for Assigning Oxidation Numbers 1 The oxidation number of any uncombined element is 0 2 The oxidation number of a monatomic ion equals the charge on the ion 3 The more electronegative element in a binary compound is assigned the number equal to the charge it would have if it were an ion 4 Oxidation Number Rules 1 A pure element has an oxidation number of 0 2 The oxidation number of an element in a monatomic ion equals the charge of the ion 3 The sum of the oxidation number of all the elements in a compound equals 0 Microsoft Word worksheet assigning oxidation numbers key docx Created Date 1 20 2015 7 32 00 PM
More picture related to Assigning Oxidation Numbers Worksheet 1
Oxidation Numbers
https://s3.studylib.net/store/data/007610548_2-cfe9ca97347fa08110828e529eaacf6d.png

Oxidation Reduction Worksheet Answers
https://media.cheggcdn.com/media/874/87475515-ed75-4017-bf20-b8879396dc53/phpePYrdh.png
/GettyImages-656142294-4bd1ea2e79ca4b59a9180f83fcf7fdd1.jpg)
Rules For Assigning Oxidation Numbers
https://www.thoughtco.com/thmb/j7-ceiFv8XH42egRPFWpIhd_k-4=/2053x1461/filters:fill(auto,1)/GettyImages-656142294-4bd1ea2e79ca4b59a9180f83fcf7fdd1.jpg
Assigning Oxidation Numbers Chemistry Tutorial Skip to main content General Chemistry Start typing then use the up and down arrows to select an option from the list Worksheet The Atom 9m Subatomic Particles 15m Isotopes 17m Ions 27m Atomic Mass 28m Periodic Table Classifications 11m Complete the examination by yourself and hand it in to receive credit Purpose This exercise is designed to teach the student how to assign oxidation numbers Oxidation numbers are very important and are used for 1 naming compounds 2 balancing oxidation reduction reactions 3 calculations in electrochemistry and other areas of chemistry
Oxidation number of each Cl goes from 0 to 1 making this a reduction oxidation 0 0 3 1 2Fe 3Cl2 6 2FeCl3 reduction Key Questions 1 Assign the oxidation numbers of each element in the following chemical species HCl H2O NH3 NO3 K 2Cr2O7 Hg2Cl2 HgCl2 Al OH 3 Na3PO4 1We will discuss electronegativity in more detail later For Rules for assigning oxidation numbers 1 The oxidation number of an element is zero 2 For a monatomic ion the oxidation number is the charge on the ion 3 The oxidation number of combined hydrogen is usually 1 4 The oxidation number of combined oxygen is usually 2 5 The sum of all oxidation numbers of atoms in a compound is zero

Balancing Equations Using Oxidation Numbers YouTube
https://i.ytimg.com/vi/Q92HA22zumU/maxresdefault.jpg

Assigning Oxidation Numbers Worksheet Answer Key Worksheet Resume
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/03/assigning-oxidation-numbers-worksheet-answer-key.jpg
Assigning Oxidation Numbers Worksheet 1 - Chemistry questions and answers Worksheet ASSIGNING OXIDATION NUMBERS Oxidation Number Rules 1 The oxidation number of any pure element is 0 2 The oxidation number of a monatomie ion equals that charge on the ion 3 The more electronegative clement in a binary compound is assigned the number oqual to the charge it would have if it were an