Worksheet Le Chatelier S Principle Le Chatelier s Principle Worksheet 1 For the reaction below which change would cause the equilibrium to shift to the right CH4 g 2H2S g CS2 g 4H2 g a Decrease the concentration of dihydrogen sulfide b Increase the pressure on the system c Increase the temperature of the system
CHEMISTRY 12 LE CH 194 TELIER S PRINCIPLE WORKSHEET 1 1 State Le Ch 226 telier s Principle 2 marks When a stress is imposed to a system at equilibrium the system will shift to oppose the stress and re establish equilibrium 2 For the reaction PCl3 g Le Chatelier s Principle Worksheet Name Date Block 1 State Le Chatelier s Principle Give an example in your answer 2 In order to decide what effect a change in total pressure will have on an equilibrium system with gases what is the first thing you
Worksheet Le Chatelier S Principle
Worksheet Le Chatelier S Principle
https://classworkassignmentanswers.weebly.com/uploads/7/9/5/5/79550400/img004-li_orig.jpg

Review Of Worksheet Le Chatelier S Principle References Alec Worksheet
https://i2.wp.com/nancyhbrim.files.wordpress.com/2013/03/lechatgrid.jpg
Le Chatelier s Principle Sample Exercises
https://imgv2-1-f.scribdassets.com/img/document/18435145/original/2bc8540970/1581005841?v=1
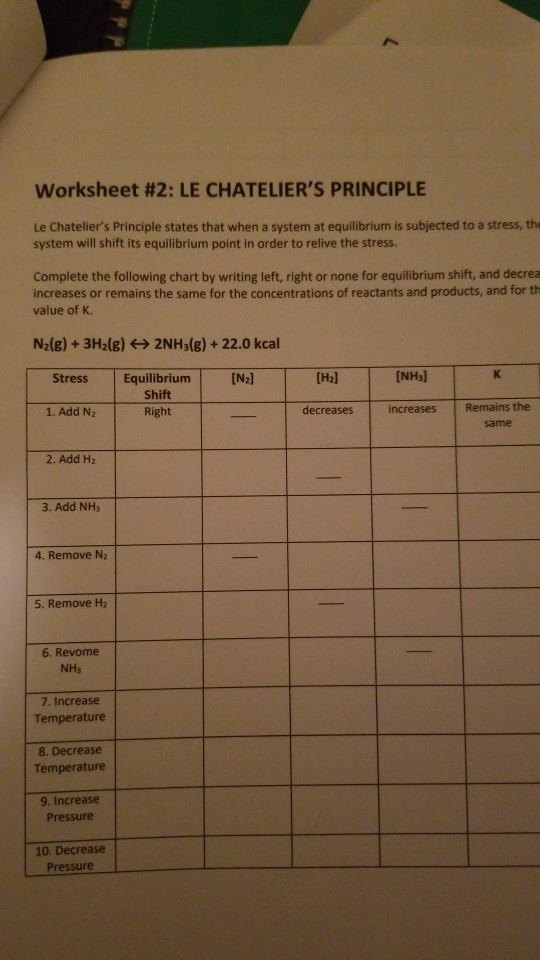
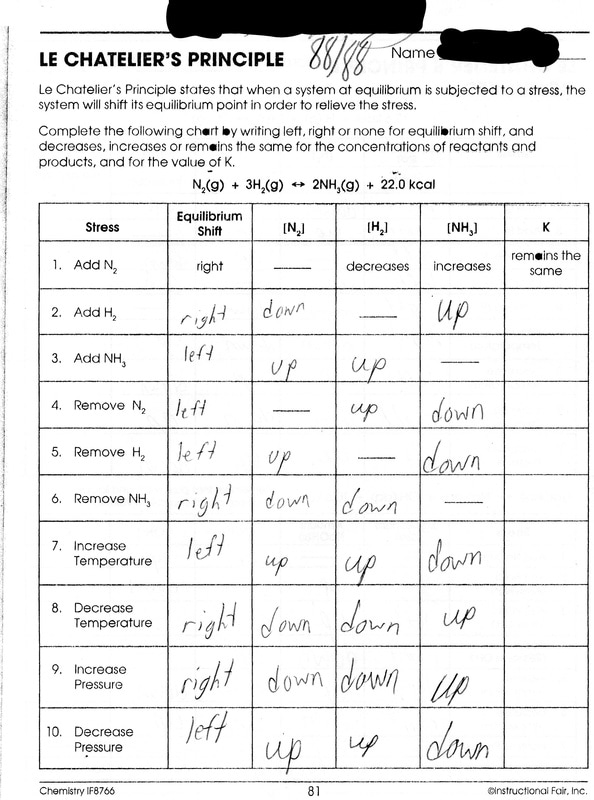
This worksheet aims to enhance their understanding of how chemical reactions reach equilibrium and how Le Chatelier s Principle elucidates their dynamic behavior Suitable for Grade 9 Grade 10 Grade 11 and Grade 12 Worksheet 2 LeChatelier s Principle Describe the changes that occur after each stress is applied to the equilibrium N 2 g 3H 2 g 2NH 3 g 92 KJ Shifts Shifts to the Stress N 2 H 2 NH 3 Right or Left Reac Prod 1 N 2 is
A stress is defined as any change which could affect the of either or both the forward and or reverse reaction When because of an applied stress the forward reaction is faster than the reverse reaction the system is said to shift to the right left Le Chatelier s Principle Worksheet 2 1 In the following reaction will the H 2 increase or decrease when equilibrium is reestablished after these stresses are applied N 2 g 3 H 2 g 2 NH 3 g 22 kJ NH 3 g is added N 2 g is removed pressure is increased Temperature is increased
More picture related to Worksheet Le Chatelier S Principle
Le Chateliers Principle Worksheet Chart Answers Get Images
https://d2vlcm61l7u1fs.cloudfront.net/media/1b4/1b46999a-c245-49ee-8dcf-8e43d6d4deec/image

Mr Brueckner s AP Chemistry Blog 2016 17 February 2017
http://3.bp.blogspot.com/-DCdgkX4qUdM/UwQgR1tjPBI/AAAAAAAAA0I/6QsFBJsHhd0/s1600/LeChat+Images+2_0001.jpg
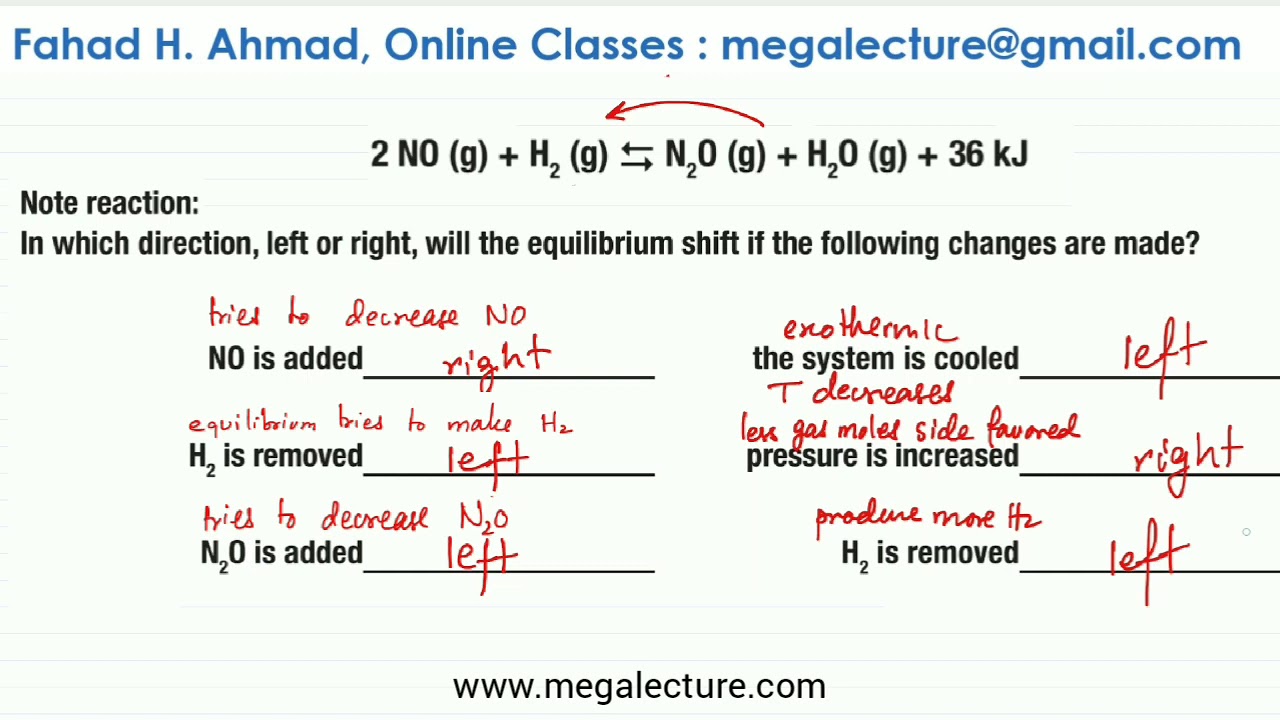
Le Chatelier s Principle Worksheet Solved Questions Mega Lecture
https://i.ytimg.com/vi/QvJTRfgMrdE/maxresdefault.jpg
Practice Worksheet Le Chatelier s Principle Match the change to the equilibrium system below with the letter of the appropriate response Each letter can be used once more than once or not at all 2SO2 g O2 g 2SO3 g 1 O2 is added to the reaction a The equilibrium shifts to the right 2 SO3 is removed from the reaction After completing these Chemistry worksheets students will be able to predict the effect of changes in concentration pressure and temperature on equilibrium position and equilibrium constant as well as explain the alteration of equilibrium position using Le Chatelier s Principle
Equilibrium Le Chatelier s Principle Practice 1 4 State the direction in which each of the following equilibrium systems would be shifted upon the application of the following stress Then state if the concentration of the listed substance will increase or decrease The Stress Reaction Shift Right or Left X increase or decrease These notes provide a scaffolded introduction to shifting equilibrium and Le Chatelier s Principle suitable for year 11 and year 12 high school chemistry students
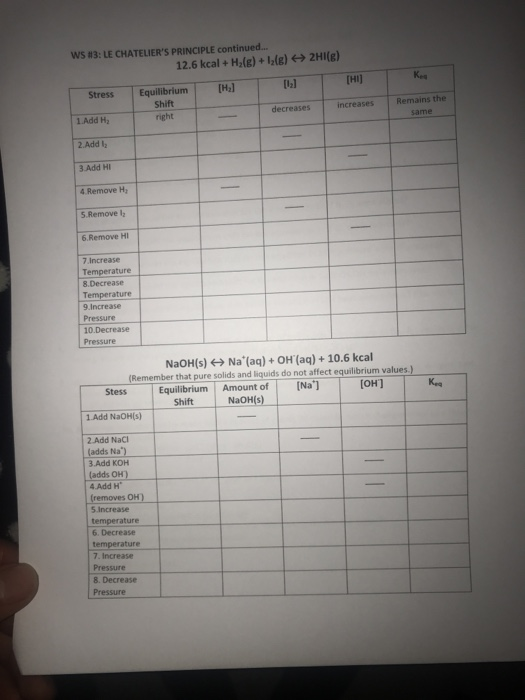
Solved Worksheet 3 LE CHATELIER S PRINCIPLE Le Chatelier s Chegg
https://media.cheggcdn.com/media/0c5/0c5b3181-5223-457e-b411-d8aadfedcb54/image.png
Chemical Systems Equilibrium Ppt Download Worksheet Template Tips And
https://d1e4pidl3fu268.cloudfront.net/d13df1dd-f09f-452e-9619-f81f5471511d/lechatelier.crop_821x616_55%2C0.preview.tiff
Worksheet Le Chatelier S Principle - Le Ch 226 telier s Principle Worksheet 1 What would happen to the position of the equilibrium when the following changes are made to the equilibrium system below CH 4 g 2H 2 S g CS 2 g 4H 2 g a Decrease the concentration of dihydrogen sulfide hydrosulfuric acid Equilibrium will shift to favor reactants