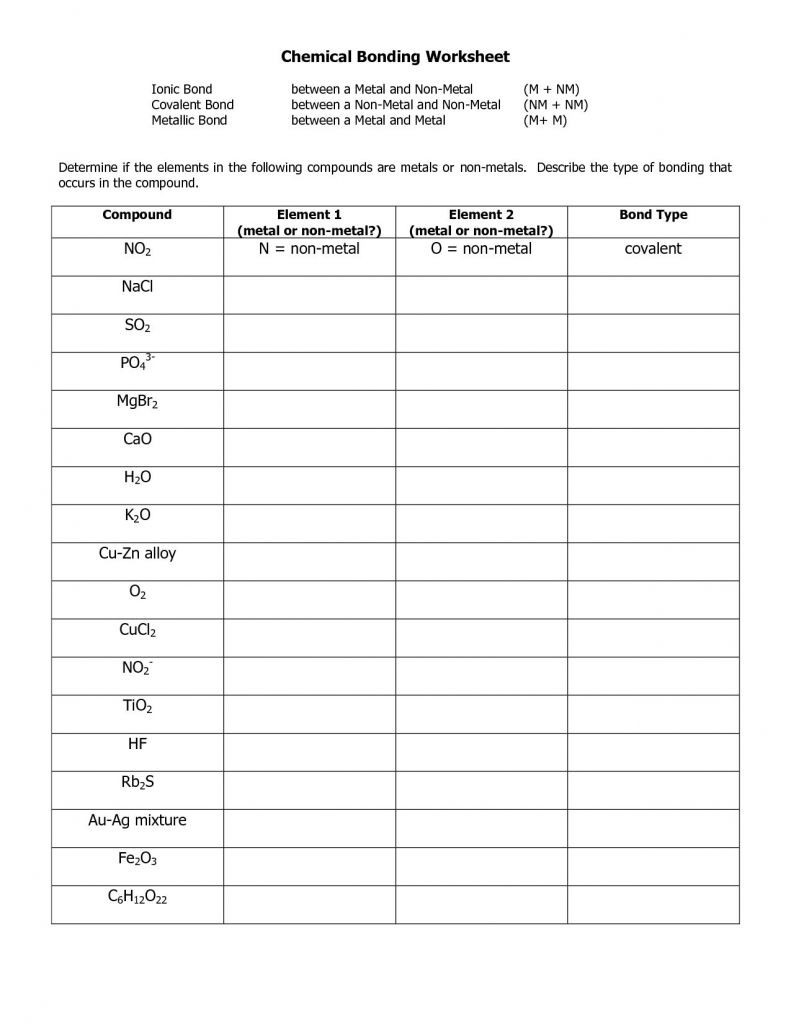
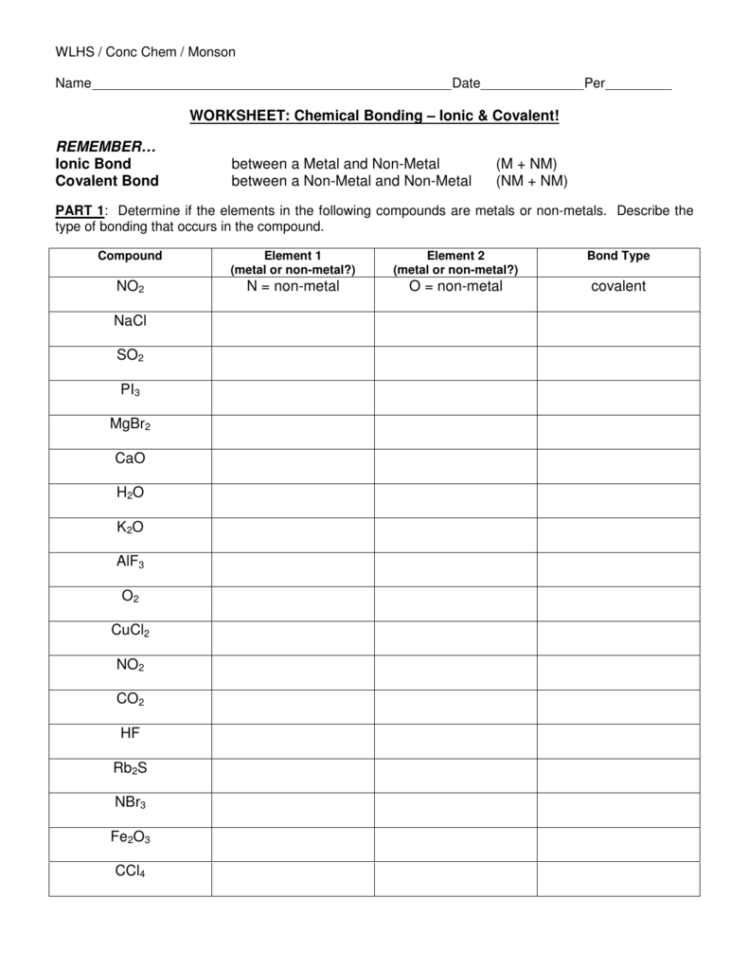
Worksheet Chemical Bonding Ionic Covalent Non metal atoms gain electrons to form anions The interactions between cations and anions are ionic and are often called ionic bonds Simply it is the coming together of opposite charges in
PART 3 Use Lewis dot structures to show the covalent bonding in the following pairs of elements Once you have determined the structure for the molecule write its structural formula in the space provided use a dash to represent a shared Fill in the table below determining if the substance is ionic or covalent If it is covalent then determine the electronegativity difference to identify if the covalent bond is polar or nonpolar
Worksheet Chemical Bonding Ionic Covalent
Worksheet Chemical Bonding Ionic Covalent
https://briefencounters.ca/wp-content/uploads/2018/11/worksheet-chemical-bonding-ionic-and-covalent-answers-part-2-with-free-worksheets-library-download-and-print-worksheets-of-worksheet-chemical-bonding-ionic-and-covalent-answers-part-2.jpg

Ionic And Covalent Bonding Worksheet
https://briefencounters.ca/wp-content/uploads/2018/11/ionic-and-covalent-bonding-worksheet-as-well-as-chemical-bonding-worksheet-key-of-ionic-and-covalent-bonding-worksheet.jpg

Lewis Dot Structure Ionic Compounds
https://sp-uploads.s3.amazonaws.com/uploads/services/1849125/20210603041942_60b8585e14074_rodrigo__kapila___chemistry___bonding___worksheet___answer_keypage2.png
Ionic bonding Ionic bonds occur between metals and non metals Electrons are transferred In order for atoms to gain a full outer shell they need to either transfer their electrons to another Learning Objectives Be able to define covalent bonds polar covalent bonds ionic bonds electronegativity dipoles formal charge molecular formula structural formula and electron dot
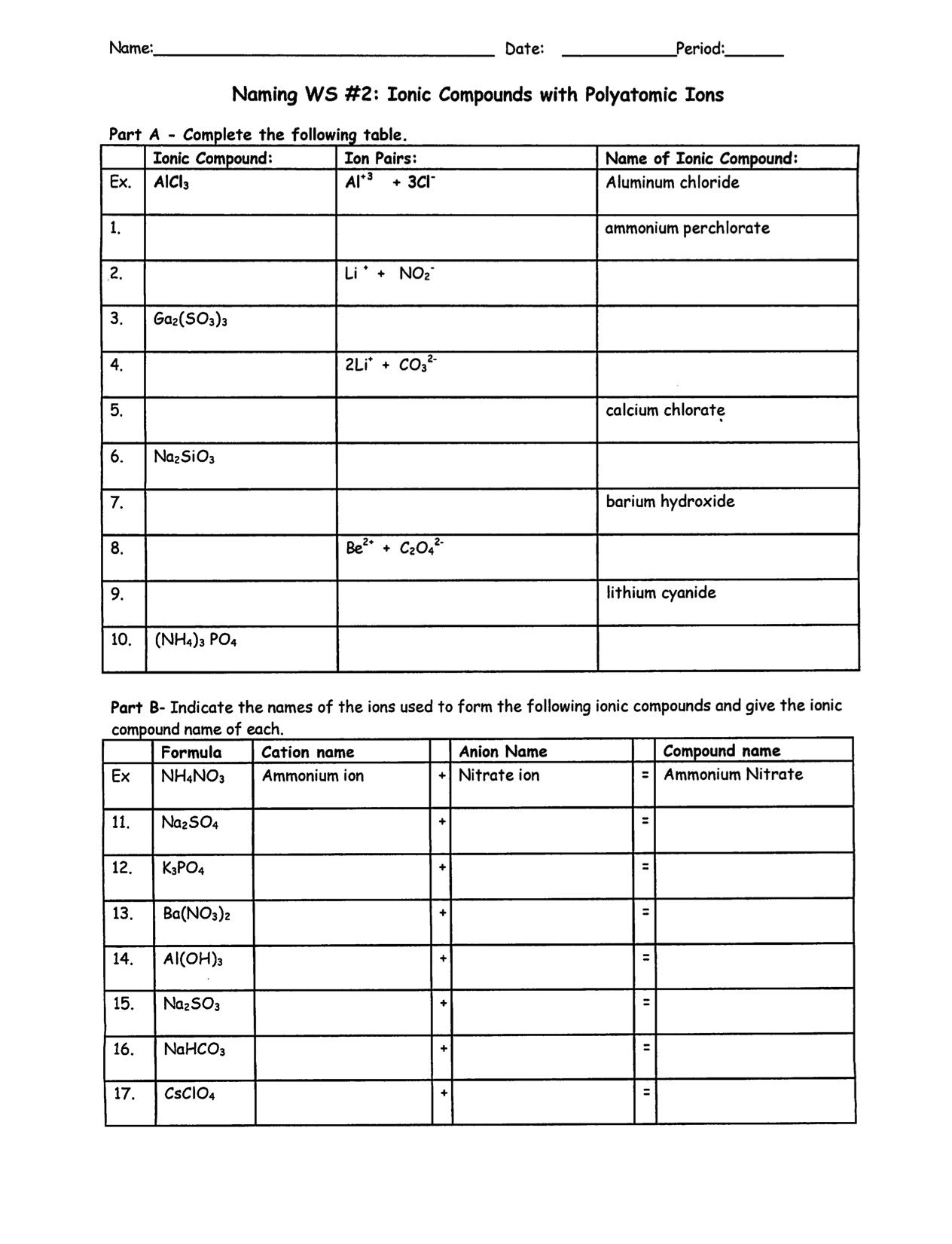
1 How are ionic bonds and covalent bonds different 2 Describe the relationship between the length of a bond and the strength of that bond 3 Identify the type s of bond s found in the PART 1 Determine if the elements in the following compounds are metals or non metals Describe the type of bonding that occurs in the compound PART 2 Use Lewis dot structures
More picture related to Worksheet Chemical Bonding Ionic Covalent
Worksheet Chemical Bonding Ionic And Covalent Also Chemical Bonds
https://www.semesprit.com/wp-content/uploads/2018/08/worksheet-chemical-bonding-ionic-and-covalent-also-chemical-bonds-of-worksheet-chemical-bonding-ionic-and-covalent.jpg
Ionic And Covalent Bonding Worksheet Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/ionic-and-covalent-bonding-worksheet-answer-key-5.jpg

Worksheet Chemical Bonding Ionic And Covalent
http://briefencounters.ca/wp-content/uploads/2018/11/worksheet-chemical-bonding-ionic-and-covalent-answers-part-2-along-with-ionic-bonding-practice-worksheet-answers-luxury-ionic-bonding-of-worksheet-chemical-bonding-ionic-and-covalent-answers.jpg
PART 2 Use Lewis dot structures to show the ionic bonding in the following pairs of elements Show the transfer of electrons using arrows Write the correct chemical formula for the ionic This resource features three different versions of a worksheet on bonding scaffolded partially scaffolded and unscaffolded Additional scaffolded worksheets look at covalent bonding ionic bonding and metallic bonding individually
CHEM1611 Worksheet 3 Ionic and Covalent Bonding Model 1 Ionic Bonding The compounds formed by metals and non metals contain ionic bonds Metal atoms lose electrons to form Developing understanding of covalent bonding Support learners to visualise covalent structures using a variety of different diagrams and physical models This worksheet is part of
Ionic And Covalent Bonding Worksheet With Answers Db excel
https://db-excel.com/wp-content/uploads/2019/09/worksheet-chemical-bonding-ionic-covalent-remember-6-749x970.png

IGCSE Identifying Ionic Covalent Bonds Teaching Resources
https://s-media-cache-ak0.pinimg.com/originals/ca/b8/f5/cab8f574367db07730ac68b13af7ba2e.jpg
Worksheet Chemical Bonding Ionic Covalent - The document is a chemistry worksheet about ionic and covalent bonding It contains questions to determine if elements are metals or non metals and the type of bonding in